- Title
-
The homeobox gene irx1a is required for the propagation of the neurogenic waves in the zebrafish retina
- Authors
- Cheng, C.W., Yan, C.H., Hui, C.C., Strähle, U., and Cheng, S.H.
- Source
- Full text @ Mech. Dev.
|
Developmental expression of irx1a in the zebrafish retina. (A–C) Coronal sections of a 48 hpf zebrafish head revealed irx1a expression in the GCL of the retina (A), but no retinal expression of irx1b or irx7 (B,C). (D) irx1a and isl-1 are co-expressed in the GCL at 48 hpf. (E–L) Lateral views of the dissected eyes at 24 hpf (E,I), 28 hpf (F,J), 32 hpf (G,K) and 36 hpf (H,L). Similar to the expression of ath5 (I–L), irx1a expression is initiated in the ventronasal retina adjacent to the choroid fissure (E) and spreads throughout the retina in a wave-like fashion (F–H). Asterisks (*) indicate the position of the choroid fissure. |
|
Ocular defects in irx1a morphants. (A,E) Dorsal view of the head region. Embryos were raised in a bright environment until 96 hpf. irx1a morphants (E) showed darker pigmentation than control morphants (A). (B,F) Lamination defects of irx1a morphant retinas revealed by propidium iodide staining. In the control morphant (B), the retina is segregated into three distinct nuclear layers and two plexiform layers. Large retinal ganglion cells can be found in the GCL, which project axons to the optic stalk (white arrowheads). In contrast, the laminar structure was disrupted and cells were uniform in size in the irx1a morphant retinas (F). By 48 hpf, pax6 expression is restricted to the GCL and the proximal INL of the control retinas (C), whereas pax6 is expressed in the entire irx1a morphant retina (G). (D,H) Lateral view of 48 hpf eye, anterior to the right, rx1 is expressed in CMZ in control retina (D) but is expressed in the entire irx1a morphant retina (H). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Black arrowheads indicate the position of the optic stalk. Asterisks (*) indicate the position of the choroid fissure. |
|
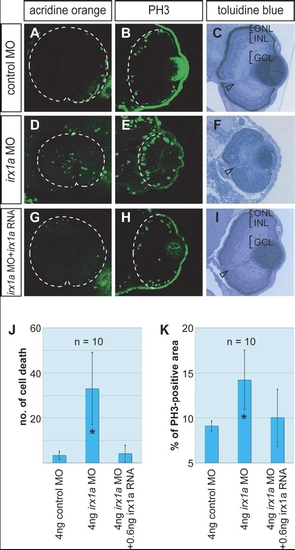
Alterations of cell proliferation, cell death and lamination in irx1a morphant retina. (A–C) Embryos injected with control MO; (D–F) embryos injected with irx1a MO; (G–I) rescue embryos injected with irx1a MO and irx1a mRNA. (A,C,G) Acridine orange fluorescent staining (lateral view of 48 hpf embryos, anterior to the left) showed that irx1a morphant retina had ectopic cell death in compare to control or rescue embryos. t-Test analysis show that the number of cell death in morphant is significant higher (J, *P≤0.005) than control. (B,E,H) Anti-phosphohistone H3 immunostaining for mitotic cells (Coronal section of 48 hpf embryos, ventral to the bottom). The ratio of proliferating cells in irx1a morphant retina (E) was significant higher (K, t-test, *P≤0.005) than control retina (B) but the rescue embryos (H) was comparable to the control. In 96 hpf, the lamination of the retina in irx1a morphant embryos (F) was disrupted but rescue embryos (I) showed normal lamination as control embryos (C). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Open arrowheads indicate the position of the optic stalk. PHENOTYPE:
|
|
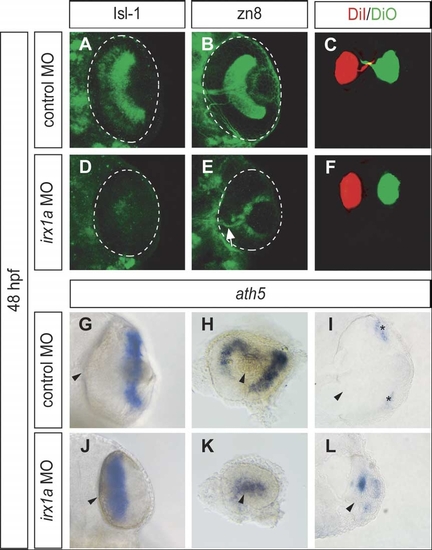
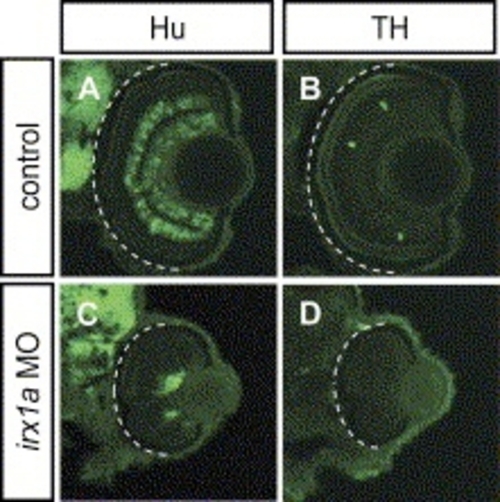
Ventral view of whole-mount immunostaining with RGC markers Isl-1 (A,D) and zn8 (B,E) in 48 hpf retinas. Less Isl-1 positive RGCs were found in the irx1a morphant retina (D) in comparison to the control (A). (B) Zn8 immunoreactivity was observed on the surface of RGC and axons in the optic nerve. However, zn8 staining was barely detectable (white arrow) in the irx1a morphant retina (E). (C, F) Dorsal view of DiI-(red) and DiO-(green) injected eyes at 48 hpf. The RGC axons were retrogradely labeled in the control retina (C). No RGC axon projection could be clearly detected in irx1a morphants (F). Expression of ath5 is altered in irx1a morphant retinas; (G,J) ventral view, (H,K) lateral view, anterior to the left, and (I,L) coronal section. At 48 hpf, ath5 is expressed in the ciliary margin (asterisks) of the control retina (G-I), whereas it is expressed near the optic stalk in the irx1a morphant retina (J–L). |
|
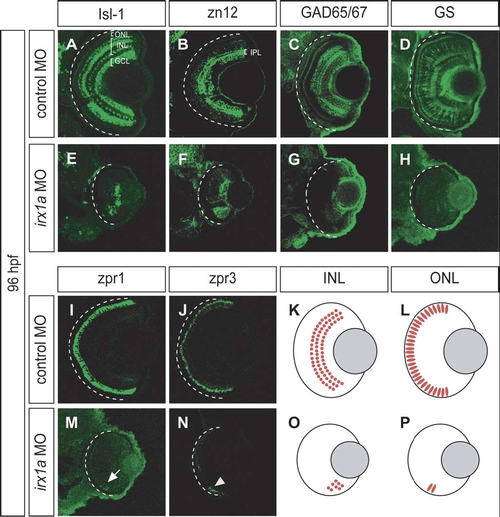
irx1a is required for neurogenesis of the INL and ONL. (A–J,M,N) Coronal sections of 96 hpf retina. Ventral to the bottom. (A,E) Isl-1 immunostaining of a control retina (A) labeled the RGCs, a subset of amacrine cells in the proximal INL, and a subset of bipolar cells in the distal INL. Only a small patch of Isl-1 positive cells could be detected in the ventral INL of the irx1a morphant retina (E). (B,F) Zn12 (HNK-1) immunostaining labeled the RGCs and amacrine cells in the INL of the control retina (B) but only detected a ventral patch of cells in the irx1a morphant retina (F). IPL, inner plexiform layer. (C,G) GAD 65/67 immunostaining labeled a subset of amacrine cells (C) in the INL but only detected some cells in the ventral side of the irx1a morphant retina (G). (D,H) Glutamine synthetase (GS) immunostaining labeled all Müller cells. In control retinas, Müller glial cells could be found in the INL and the processes are extended through the entire retina (D). In irx1a morphant retinas, Müller glial cells were completely missing (H). (I,M) Zpr1 immunostaining of a control retina (I) labeled a subset of cone photoreceptors in the ONL. In the irx1a morphant retina (M), only a small patch of zpr1 labeled cells (white arrow) could be detected on the ventral side. (J,N) Zpr3 immunostaining of a control retina (J) labeled rod photoreceptors in the ONL. Similar to zpr1 staining, a small patch of zpr3-expressing cells (white arrowhead) could be detected in the ventral aspect of the irx1a morphant retina (N). (K,L,O,P) Schematic representation of the retinal defects of irx1a morphants. Neurogenesis is initiated in a ventronasal patch but does not spread through the retina in the irx1a morphants. (O, P). |
|
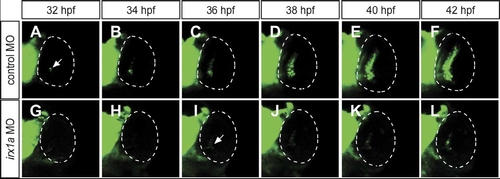
Spread of the waves of shh-GFP expression is dependent on irx1a. In vivo time-lapse recording the eyes of a live embryo carrying the shh-GFP transgene, ventral view, anterior to the top. In control embryos (A–F), shh-GFP is expressed in the ventronasal side of the control retina (A), its expression then spreads to the central retina at about 36 hpf (C), and to the entire GCL and INL at about 42 hpf (F). The expression of shh-GFP is initiated in the irx1a morphant retina (I, arrow) with a delay of about 4 hrs. In contrast to the wildtype, GFP is not expressed in the central and dorsal regions of the irx1a morphant retina (G–L). Note both the GCL and INL shh-GFP waves are blocked in irx1a morphant. |
|
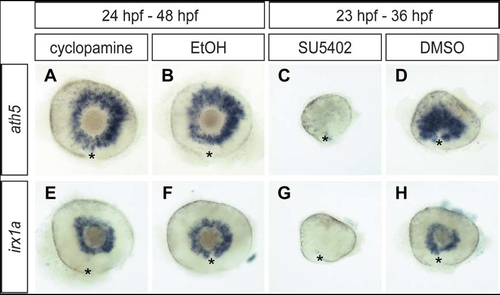
Irx1a expression is depended on Fgf signaling but not shh signaling. Lateral view of 48 hpf (A,B,E,F) and 36 hpf (C,D,G,H) retinas. Shh signaling inhibitor cyclopamine treated (24–48 hpf) embryos (A,E) showed comparable ath5 and irx1a expression as the mock solution treated controls (B,F). In contrast, both ath5 and irx1a expression were almost absent in Fgf signaling inhibitor SU5402 treated (23–36 hpf) embryos (C,G) in compare to the control (D, H). Asterisks (*) indicate the position of the choroid fissure. |
|
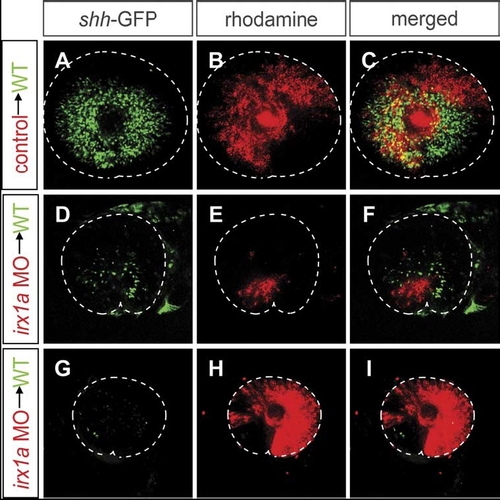
The effect of irx1a knockdown on shh-GFP expression is both cell-autonomous and non-cell-autonomous. Lateral view of 48 hpf retina. Ventral to the bottom, anterior to the left. Blastomere transplantations were carried out to determine the cell-autonomy of the effects of irx1a knockdown. The donor embryos, which carry the shh-GFP transgene, were labeled with rhodamine-dextran. Donor cells were transplanted into wild-type shh-GFP hosts at blastula stages. The shh-GFP expression in retinas with transplanted irx1a-MO cells was severely reduced (D–I), while control-MO injected donor cells had no effect on shh-GFP expression (A–C). Transplanted cells failed to express the shh-GFP transgene in all cases (red stain panels D–F) suggesting a cell-autonomous requirement of irx1a. However, shh-GFP expression was also reduced in areas that were not occupied by irx1a-MO containing cells. Host retinas with large irx1a-MO clones (G–I) lacked almost entirely GFP expression in areas derived from host cells. These results indicate an additional non-cell-autonomous effect of irx1a knockdown. A microphthalmia phenotype was also observed in the irx1a-MO transplanted hosts with large clones of irx1a-MO cells. |
|
PHENOTYPE:
|
Reprinted from Mechanisms of Development, 123(3), Cheng, C.W., Yan, C.H., Hui, C.C., Strähle, U., and Cheng, S.H., The homeobox gene irx1a is required for the propagation of the neurogenic waves in the zebrafish retina, 252-263, Copyright (2006) with permission from Elsevier. Full text @ Mech. Dev.