FIG 2
|
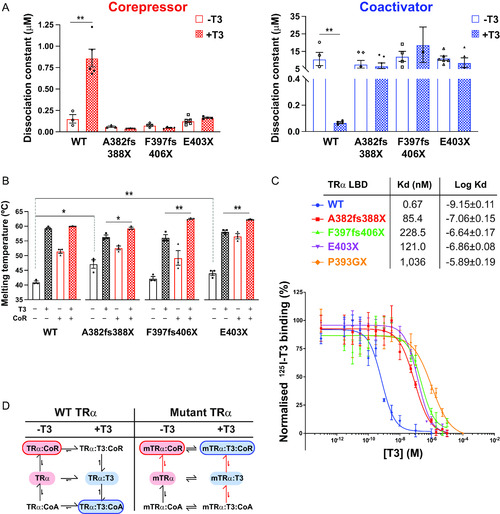
Interaction of human TRα mutants with corepressor and coactivator peptides (A) Binding affinities (dissociation constants) of wild-type and mutant TRα LBDs for corepressor in the absence (−T3) and presence (+T3) of T3 and for coactivator in the absence and presence of T3 in fluorescence anisotropy assays. Error bars indicate mean ± SEM (n = 5); **, P < 0.01. (B) Thermal stability (melting temperature) of wild-type and mutant TRα LBDs alone, in the presence of T3, with SMRT corepressor peptide, or with both SMRT and T3. Error bars indicate mean ± SEM (n = 5); *, P < 0.05; **, P < 0.01. (C) Radiolabeled T3 competitive binding assays of wild-type, mutant, and artificial mutant TRα showing the dissociation curves in the presence of increasing concentrations of unlabeled T3, and the dissociation constant (Kd) obtained. Data presented as mean ± SEM from two independent experiments performed in triplicate. (D) Scheme summarizing how the patient-derived mutations perturb the equilibria between ligand- and coregulator-bound species. |