FIG 7
|
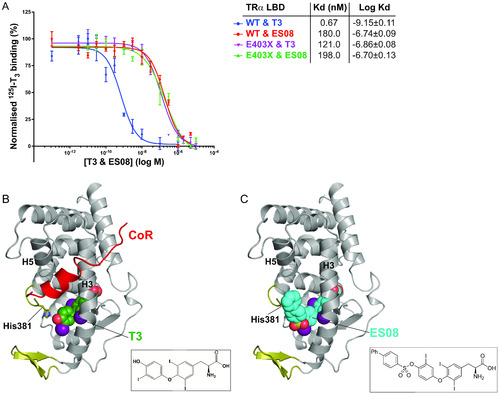
Binding affinity and fitting of ES08. (A) Competitive binding assay with radiolabeled T3, wild-type or mutant TRα, and increasing concentrations of unlabeled ES08, indicating dissociation constants (Kd) obtained. Data shown are the mean ± SEM from two independent experiments performed in triplicate. Note that the data for WT binding T3 is included for comparison, duplicated from Fig. 2C. (B) Model of corepressor binding to P393GX:T3 complex. T3 is shown in green with the SMRT corepressor peptide in red. Interaction of the LBD and corepressor peptides is mediated by corepressor recruitment in a hydrophobic groove formed by H3 and H5 of the LBD. Due to the lack of H12 in TRα mutants, there is no disruption of the CoR binding surface, and the corepressor remains interacting with the mutant receptors even in the presence of T3 forming the ternary complex. (C) Model of ES08 (cyan) binding to P393GX. Here, in contrast to T3, the extension at the 4′-hydroxyl of the outer thyronine ring potentially disrupts the CoR binding surface of the LBD and, consequently, ES08 interferes with corepressor interaction. |