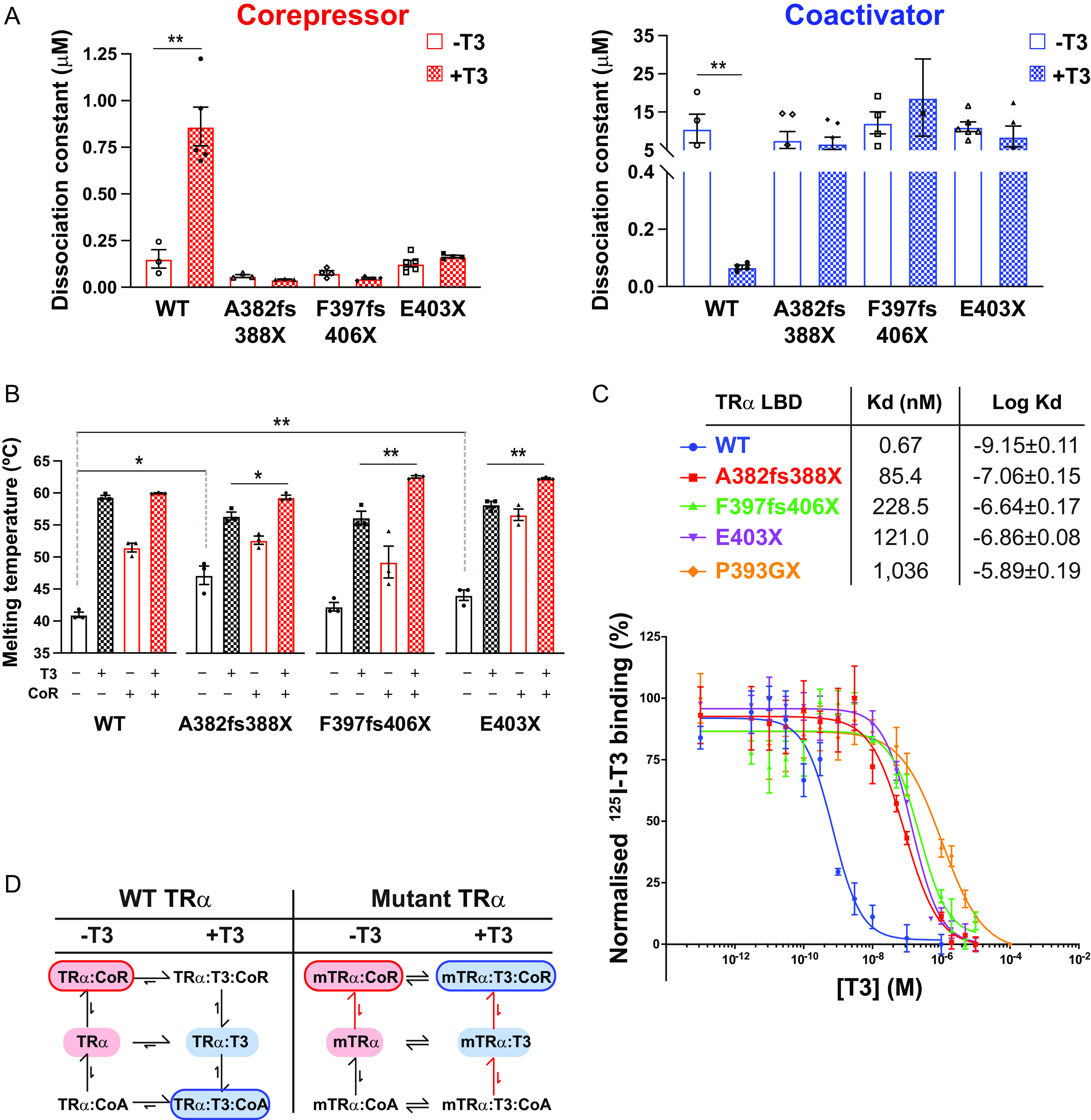
FIG 2 Interaction of human TRα mutants with corepressor and coactivator peptides (A) Binding affinities (dissociation constants) of wild-type and mutant TRα LBDs for corepressor in the absence (−T3) and presence (+T3) of T3 and for coactivator in the absence and presence of T3 in fluorescence anisotropy assays. Error bars indicate mean ± SEM (n = 5); **, P < 0.01. (B) Thermal stability (melting temperature) of wild-type and mutant TRα LBDs alone, in the presence of T3, with SMRT corepressor peptide, or with both SMRT and T3. Error bars indicate mean ± SEM (n = 5); *, P < 0.05; **, P < 0.01. (C) Radiolabeled T3 competitive binding assays of wild-type, mutant, and artificial mutant TRα showing the dissociation curves in the presence of increasing concentrations of unlabeled T3, and the dissociation constant (Kd) obtained. Data presented as mean ± SEM from two independent experiments performed in triplicate. (D) Scheme summarizing how the patient-derived mutations perturb the equilibria between ligand- and coregulator-bound species.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Mol. Cell. Biol.