- Title
-
Kit and foxd3 genetically interact to regulate melanophore survival in zebrafish
- Authors
- Cooper, C.D., Linbo, T.H., and Raible, D.W.
- Source
- Full text @ Dev. Dyn.
|
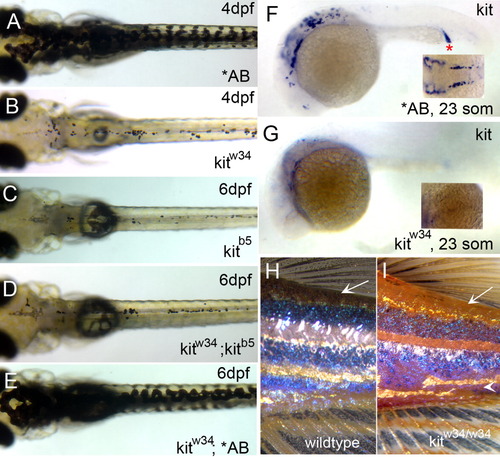
Characterization of kitw34 mutant larvae. A: AB zebrafish at 4 days postfertilization (dpf), illustrating wild-type melanophore morphology and patterning. B: kitw34 zebrafish at 4 dpf, have small, rounded melanophores. Lateral and ventral stripe melanophores are largely missing by this stage. C-E: kitw34/kitb5 complementation analysis. C: In kitb5, 6 dpf zebrafish, the majority of melanophores have died. D,E: While progeny from a homozygous kitb5 and kitw34 cross show defects in melanophore morphology and survival (D), progeny from a kitw34 and AB cross are rescued and show wild-type melanophore patterning (E). F,G: In situ analysis for kit mRNA during early melanogenesis. F: Wild-type zebrafish express kit in the head, anterior trunk and intermediate cell mass (red asterisk) at 23 somites. G: kitw34 embryos lack kit expression. H,I: kitw34 adults have a reduction in melanophores. H: Wild-type pigment pattern showing three distinct dark stripes on the flank near the dorsal and caudal fins. I: kitw34 adult showing reduced overall pigmentation, especially in dorsal areas (arrow) and throughout the dark stripes. The darks stripes are disorganized and the ventral stripe is lost with kit loss-of-function (arrowhead). EXPRESSION / LABELING:
|
|
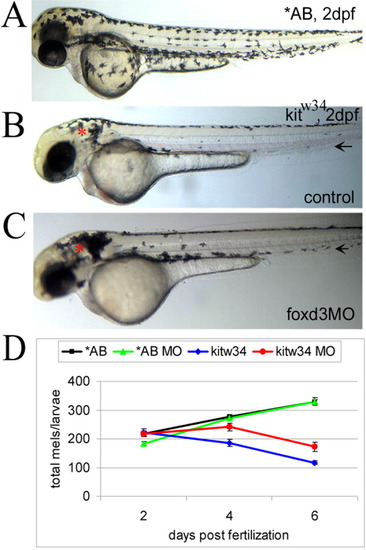
foxd3 loss-of-function reduces loss of melanophores in kitw34 zebrafish. A: In 2 days postfertilization (dpf) wild-type zebrafish (AB), melanophores have migrated extensively, anteriorly to the head and ventrally over the yolk. B: In 2 dpf kitw34 zebrafish, melanophores are specified, but largely fail to migrate from sites of origin: the dorsal trunk and area caudal to the otic vesicle (red asterisk). C: kitw34 zebrafish injected with foxd3 morpholino oligonucleotide (MO) have more melanophores, especially apparent near the otic vesicle (foxd3MO, red asterisk). Both the ventral and lateral stripes show an increase in melanophores (black arrows). D: Line graph showing counts of total melanophores in 2, 4, and 6 dpf AB or kitw34 larvae, either uninjected (control) or injected with foxd3 MO. A significant change in the number of melanophores is observed in kitw34 mutants after injection of foxd3 MO (red) as compared to kitw34 uninjected controls (blue; P < 0.0001 by two-way analysis of variance (ANOVA); 7-11 fish per time point), whereas AB foxd3 morphants (green) show no significant difference as compared to uninjected AB controls (black; P = 0.49 by two-way ANOVA; 9-13 fish per time point). |
|
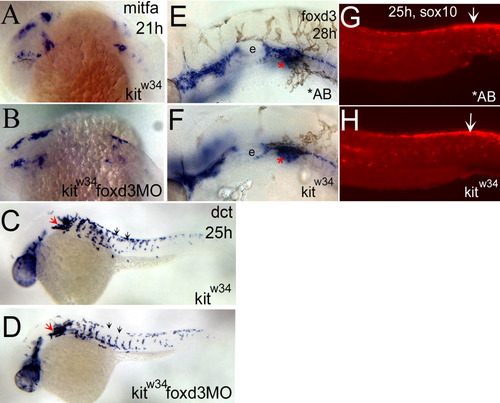
foxd3 loss-of-function does not affect the number of melanoblasts specified from kitw34 neural crest. A,B: mitfa expression is detected in the head and anterior trunk of kitw34 21 hours postfertilization (hpf) embryos. This pattern is not dramatically altered in kitw34 foxd3 morphants. C,D: In 25 hpf kitw34 embryos, dopachrome tautomerase (dct) -positive cells are found caudal to the eye and ear (red arrow), and in streams migrating ventrally between somites from the dorsal trunk (black arrows). The kitw34 foxd3 morphants show some differences in migration (compare C and D, black arrows), but the overall number of cells appears similar. E,F: At 28 hpf, AB and kitw34 embryos, processed by in situ hybridization for foxd3 message. foxd3 expression appears unchanged by kit loss-of-function. E: The developing ear or otic vesicle. G,H: At 25 hpf, AB and kitw34 embryos, processed by fluorescent in situ hybridization for sox10 message. sox10 expression in premigratory neural crest cells is mostly unchanged (white arrows), although there is some reduction in peripheral expression, consistent with the reduction in melanoblast migration observed in kitw34 mutants. EXPRESSION / LABELING:
|
|
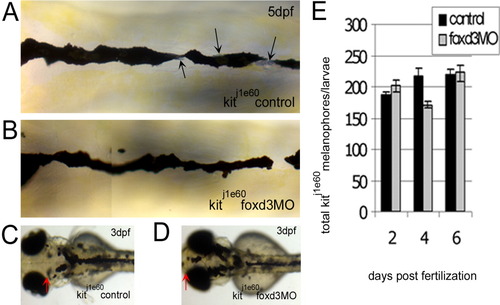
The increase in kit mutant melanophores with foxd3 loss-of-function is not due to transfating of other foxd3-dependent cell types. A-D: The kitj1e60 melanophore migration mutants were injected (or left uninjected; control) with foxd3 morpholino oligonucleotide (MO; foxd3MO), imaged at 3 (C,D) and 5 days postfertilization (dpf; A,B) and fixed at 2, 4, and 6 dpf for melanophore quantification. A: Melanophores localized to the anterior dorsal trunk display relatively normal, nonapoptotic morphology. Black arrows indicate silver pigment cells, iridophores. B: In kitj1e60 foxd3 morphants, foxd3-dependent iridophores are gone indicating MO function (average control and morphant iridophores ± standard deviation at 4 dpf are 52±3 and 18±14, respectively), yet melanophore morphology and number remain similar to uninjected controls. C: The kitj1e60 control melanophores do not localize to the anterior most portion of the head, indicating a migration defect. D: The presence of melanophores over the head is partially rescued with foxd3 loss-of-function (red arrows indicate anterior extent of melanophores). E: The kitj1e60 melanophores are not significantly increased with foxd3 loss-of-function at 2, 4, and 6 dpf (P = 0.31 by 2-way analysis of variance; 8-15 fish per time point). PHENOTYPE:
|
|
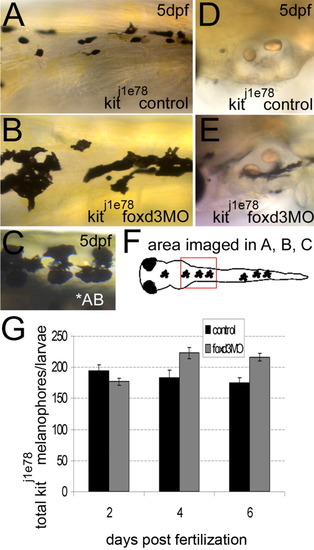
foxd3 loss-of-function improves the number and morphology of kitj1e78 survival mutant melanophores. A,B,D,E: The kitj1e78 survival mutants were injected with foxd3 morpholino oligonucleotide (MO; foxd3MO) or uninjected embryos were examined as controls. A: Control kitj1e78 melanophores are small and rounded, characteristic of apoptosing melanophores. B,C: Loss of foxd3 activity restores some wild-type morphology to kitj1e78 melanophores, and increases the number of melanophores (compare panel B to the AB wild-type melanophores in panel C). D: The kitj1e78 controls lack ventral stripe melanophores near the otic vesicle, which have apoptosed and been removed from the fish. E: The kitj1e78 foxd3 morphants show increases in melanophores at this location, suggesting increased survival. F: Cartoon illustrating the location of images taken in A-C. G: Quantification at 2, 4, and 6 days postfertilization (dpf) of kitj1e78 and kitj1e78 foxd3 morphant melanophores. There is a statistically significant increase in kitj1e78 animals after foxd3 MO injection (P < 0.005 by 2-way analysis of variance; 10-19 fish per time point). PHENOTYPE:
|
|
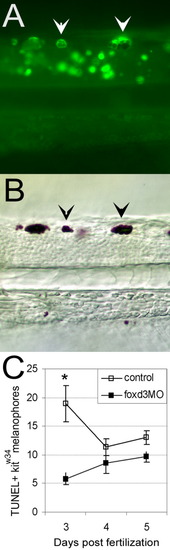
foxd3 loss-of-function partially rescues melanophores from apoptosis in kitw34 larvae. A: Three days postfertilization (dpf) kitw34 larvae analyzed by TUNEL (terminal deoxynucleotidyl transferase-mediated deoxyuridinetriphosphate nick end-labeling) assay. Examples of posterior trunk melanophores positive for TUNEL signal are marked with white arrowheads. B: Brightfield image of larvae shown in A. TUNEL-positive melanophores (black arrowheads) are distinct in size and shape. C: Line graph indicating the total number of TUNEL-positive melanophores found in the dorsal stripe of uninjected or foxd3 morpholino oligonucleotide (MO) -injected kitw34 larvae quantified at 3, 4, and 5 dpf. There is a significant decrease in TUNEL-positive melanophores at 3 dpf with foxd3 loss-of-function. (control n = 45 fish, 649 TUNEL+ cells; foxd3MO n = 64 fish, 493 TUNEL+ cells; *P < 0.001 by Bonferroni posttest at 3 dpf). PHENOTYPE:
|
|
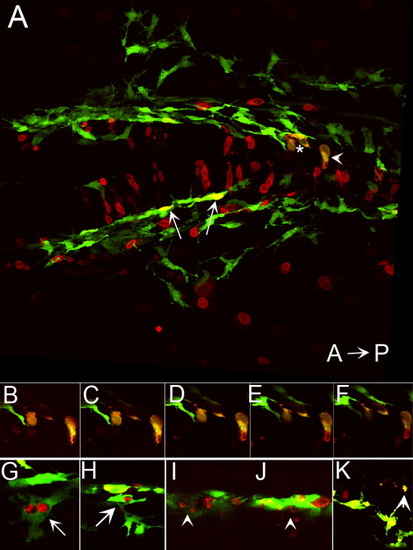
Ectopic expression of foxd3 promotes cell death in kit mutants. A: Confocal stack image showing a dorsal view of the posterior head of a 28 hours postfertilization (hpf) kitw34/w34 embryo transgenic for mi:gfp and also expressing myc-foxd3 (red) under the control of a heat shock promoter. Expression of myc-foxd3 was induced at 19 hpf. Both wild-type (arrows) and fragmenting (asterisk and arrowhead) melanophores are observed. B-F: Confocal slices showing fragmenting cells indicated by asterisk and arrowhead in A. Note the presence of Foxd3-positive fragments within the GFP+ melanophores. G,H: Confocal slices showing additional examples of GFP/Foxd3+ cells designated as normal melanophores. I-K: Confocal slices showing examples of GFP/Foxd3+ cells designated as fragmenting melanophores (arrowheads). |