- Title
-
Antioxidant, Osteogenic, and Neuroprotective Effects of Homotaurine in Aging and Parkinson's Disease Models
- Authors
- Minoia, A., Piritore, F.C., Bolognin, S., Pessoa, J., Bernardes de Jesus, B., Tiso, N., Romanelli, M.G., Schwamborn, J.C., Dalle Carbonare, L., Valenti, M.T.
- Source
- Full text @ Antioxidants (Basel)
|
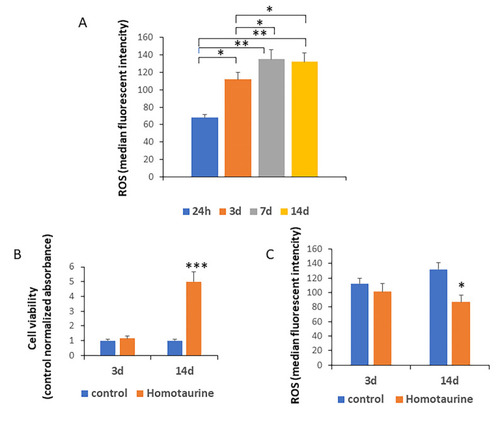
Assessment of ROS levels, MSC viability, and the effect of homotaurine treatment during a 14-day period. ( |
|
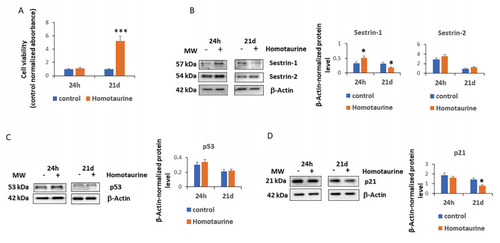
Effect of homotaurine on cell viability, sestrin expression, and cell cycle regulators in MSCs at 24 h and 21 days of treatment. ( |
|
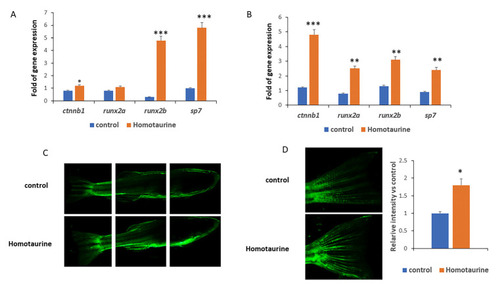
Effects of homotaurine on osteogenesis and angiogenesis gene expression in zebrafish. ( |
|
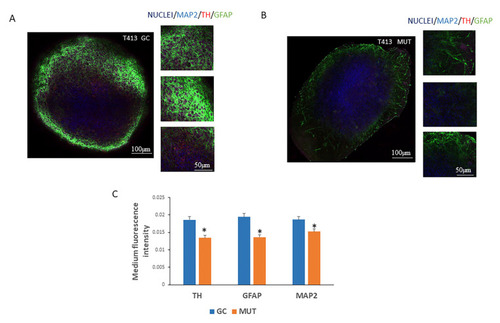
Expression of neuronal markers in 3D neuronal epithelial stem cell (NESC) organoids derived from induced pluripotent stem cells (iPSCs) carrying the leucine-rich repeat kinase 2 (LRRK2) G2019S mutation. ( |
|
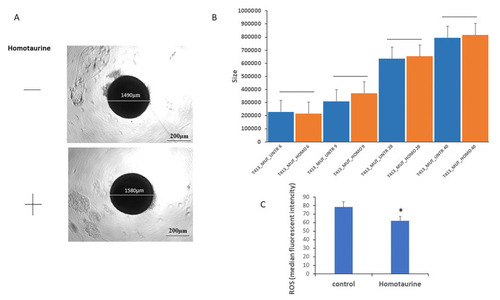
Evaluation of homotaurine’s effects on LRRK2-G2019S mutated midbrain organoids size and ROS levels during differentiation in 8 pooled organoids for each condition. ( |
|
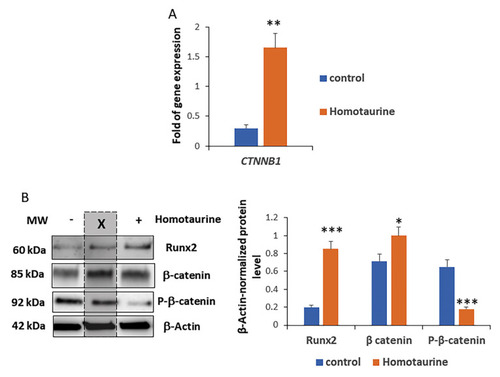
Modulation of Wnt signaling effectors by homotaurine treatment of LRRK2-G2019S-mutated midbrain organoids. ( |