- Title
-
SB218078 inhibits angiogenesis and epithelial-mesenchymal transition in breast cancer
- Authors
- Wu, Q., Xu, J., Tang, X., Yu, J., Li, B., Yang, J., Zhang, X.
- Source
- Full text @ Front Pharmacol
|
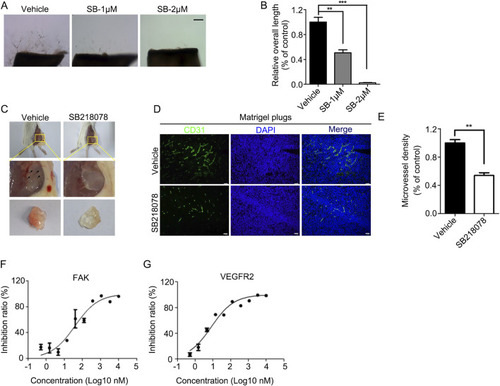
SB218078 Inhibits Vascular Development in Zebrafish Embryos. PHENOTYPE:
|
|
SB218078 inhibits angiogenesis |
|
SB218078 inhibits angiogenesis |
|
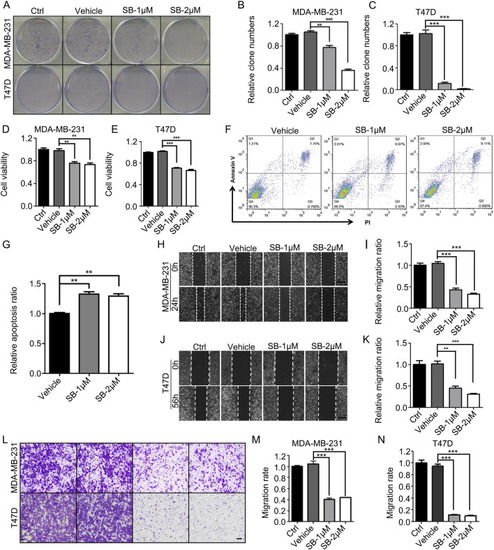
SB218078 inhibits the growth of breast cancer cells |
|
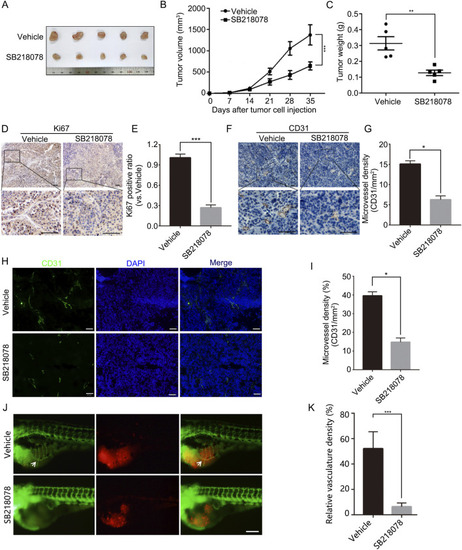
SB218078 inhibits the growth and angiogenesis of breast cancer |
|
SB218078 Inhibits the EMT in Breast Cancer Cells. |
|
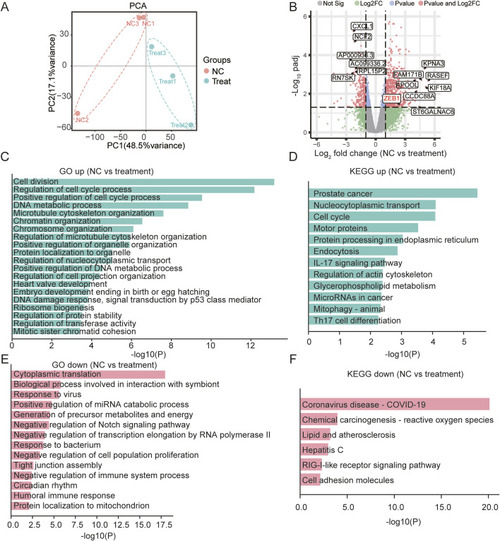
ZEB1-Independent Effects of SB218078 on Angiogenesis. |