- Title
-
Analysis of tumor-infiltrating exhausted T cells highlights IL-6 and PD1 blockade as a combined immunotherapy strategy for non-small cell lung cancer
- Authors
- Zhang, L., Guo, X., Sun, X., Liao, J., Liu, Q., Ye, Y., Yang, Z., Cressey, R., He, Q., Yuan, Q.
- Source
- Full text @ Front Immunol
|
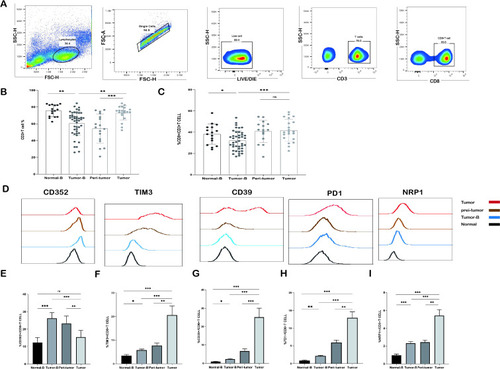
Frequency of CD3+ T cells and CD8+ T cells detected by flow cytometry. |
|
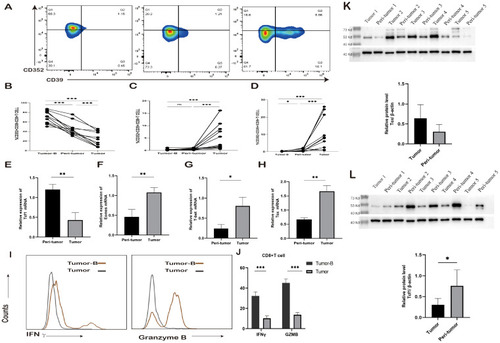
Changes in exhausted CD8+ T cell subsets. |
|
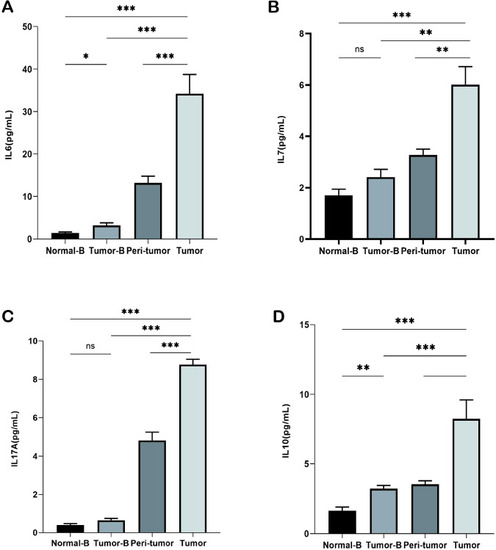
Detection of cytokines in plasma and tissue homogenization supernatants. |
|
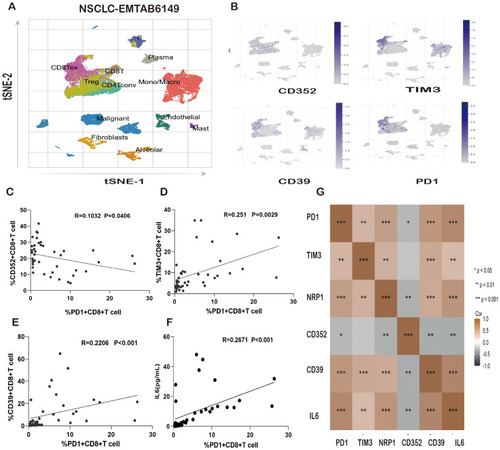
Correlation of IL-6 and PD-1 expression levels with T-cell exhaustion markers in lung cancer patients. |
|
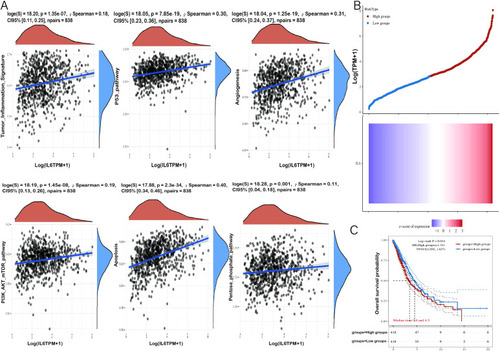
Correlation analysis of IL-6 gene with tumor-related pathways and prognosis. |
|
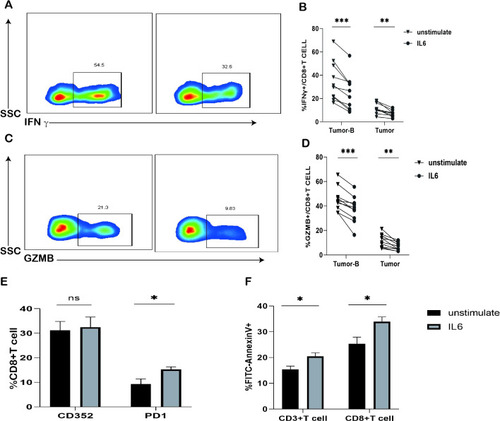
Effects of IL-6 |
|
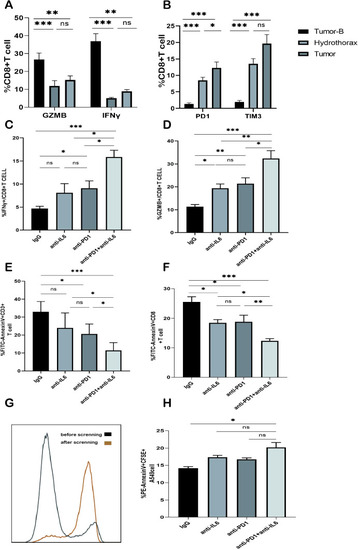
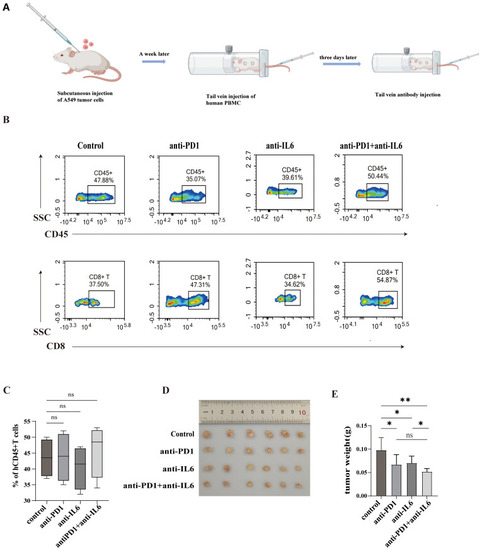
Effect of the combined blockade of PD1 and IL-6 on CD8+ T cells |
|
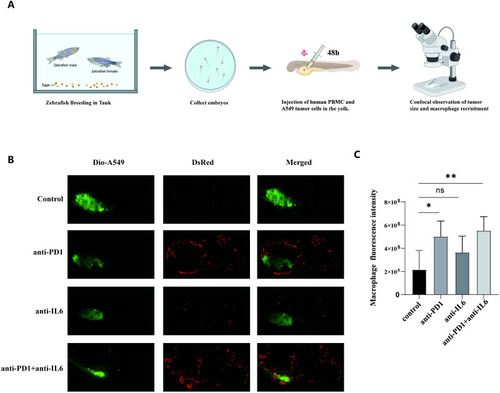
Macrophage recruitment by combination treatment with nivolumab and anti-IL-6 antibody. |
|
Combined blockade of PD1 and IL-6 on humanized mouse tumors. |