- Title
-
Receptor tyrosine kinase inhibition leads to regression of acral melanoma by targeting the tumor microenvironment
- Authors
- Smith, E.A., Belote, R.L., Cruz, N.M., Moustafa, T.E., Becker, C.A., Jiang, A., Alizada, S., Prokofyeva, A., Chan, T.Y., Seasor, T.A., Balatico, M., Cortes-Sanchez, E., Lum, D.H., Hyngstrom, J.R., Zeng, H., Deacon, D.C., Grossmann, A.H., White, R.M., Zangle, T.A., Judson-Torres, R.L.
- Source
- Full text @ J. Exp. Clin. Cancer Res.
|
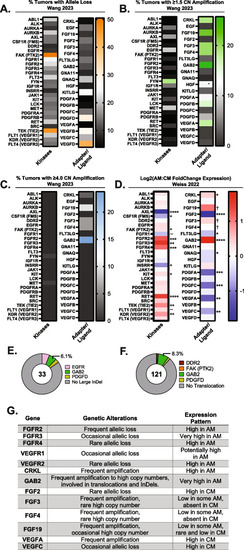
Acral melanoma (AM) tumors highly amplify and/or upregulate RTK adapter proteins, VEGFR, FGFR, and FGF ligands |
|
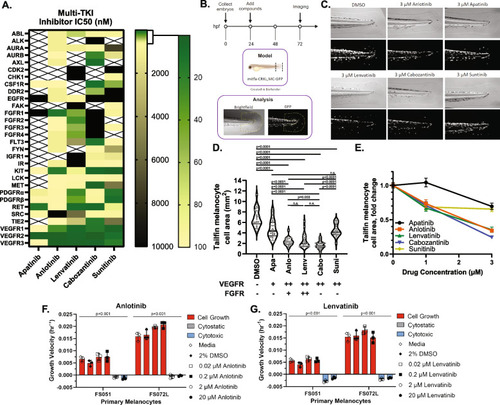
Blockade of FGFR/VEGFR receptors inhibits melanogenesis in a dose-dependent fashion. |
|
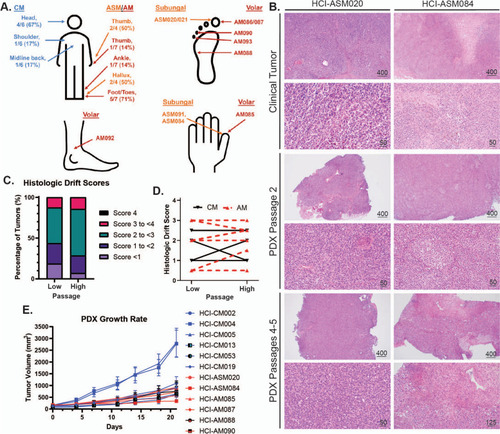
Histologic and tumor growth rate characterization of AM and CM PDX tumor models. |
|
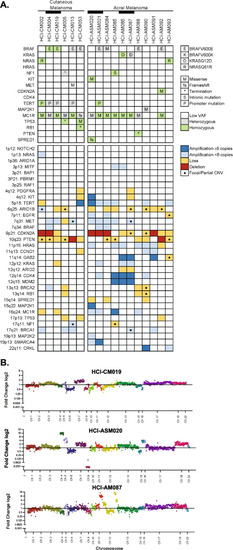
Genetic characterization of AM and CM PDX tumor models. |
|
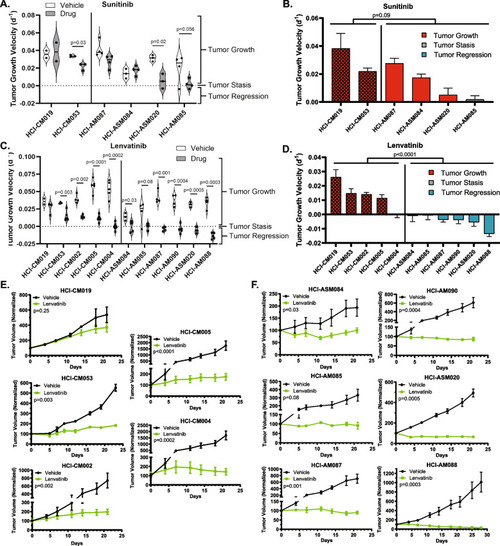
Dual FGFR/VEGFR inhibition with Lenvatinib induces tumor stasis or regression in all AM PDX tumors. Tumor growth velocity for individual Sunitinib (daily, 40 mg/kg) and Lenvatinib (daily, 50 mg/kg) treated PDX tumors are represented as violin plots ( |
|
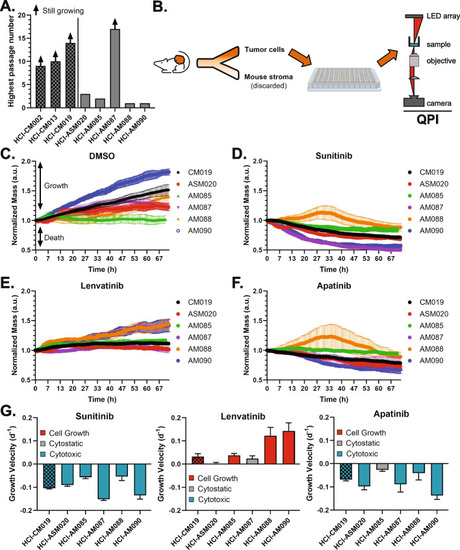
Dual FGFR/VEGFR is not directly cytotoxic in dissociated AM PDX tumor cells. |
|
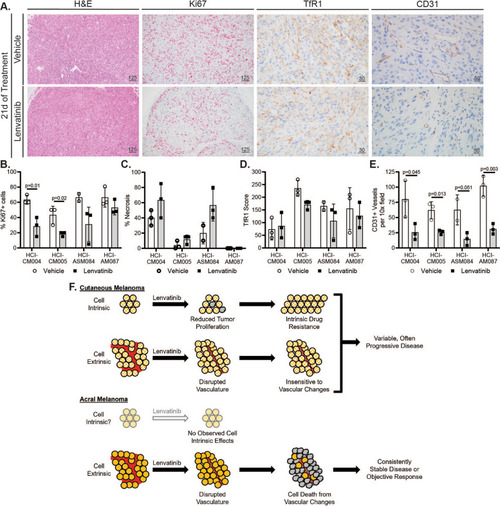
Lenvatinib halts AM tumor growth or induces regression by remodeling tumor vasculature. |