- Title
-
The oncogenic ADAMTS1-VCAN-EGFR cyclic axis drives anoikis resistance and invasion in renal cell carcinoma
- Authors
- Wen, Y.C., Lin, Y.W., Ho, K.H., Yang, Y.C., Lai, F.R., Chu, C.Y., Chen, J.Q., Lee, W.J., Chien, M.H.
- Source
- Full text @ Cell Mol. Biol. Lett.
|
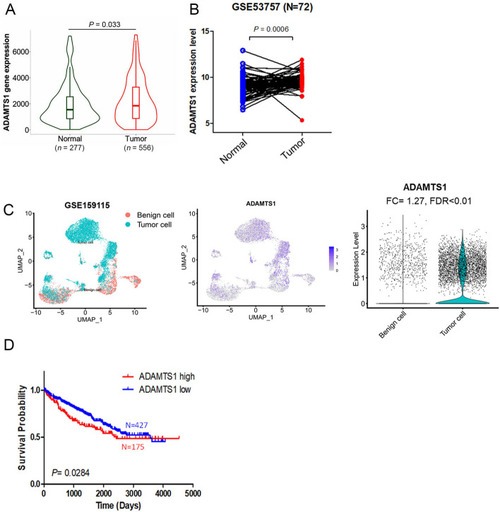
Elevated ADAMTS1 expression in renal cell carcinoma (RCC) tissues is associated with a poor prognosis. |
|
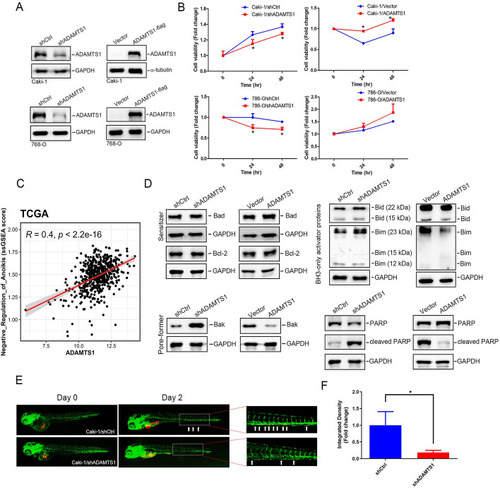
ADAMTS1 expression promotes anoikis resistance of renal cell carcinoma (RCC) via inhibiting Bid, Bim, and Bak. |
|
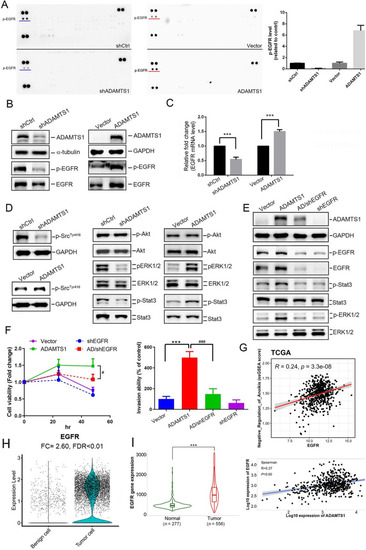
ADAMTS1 expression activates epidermal growth factor receptor (EGFR) signaling cascades to promote anoikis resistance of and invasion by Caki-1 renal cell carcinoma (RCC) cells. |
|
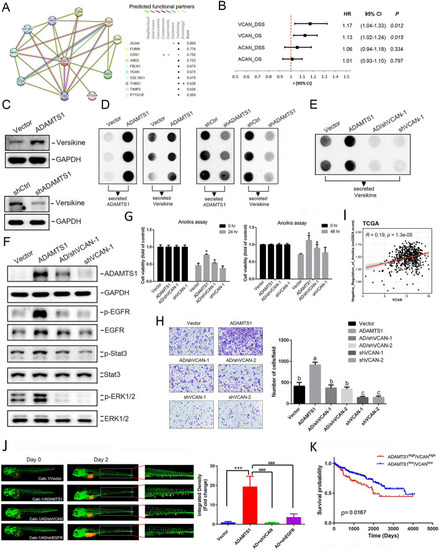
Versican (VCAN) cleavage is critical for ADAMTS1-mediated epidermal growth factor receptor (EGFR) activation, anoikis resistance, invasion, and metastasis of renal cell carcinoma (RCC) cells. |
|
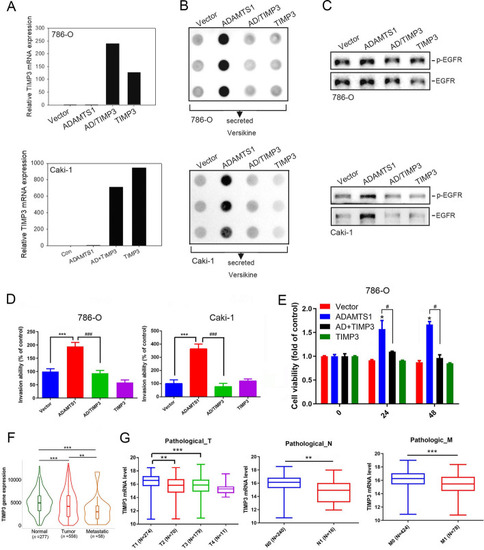
The metalloproteinase activity of ADAMTS1 is pivotal in stimulating the secretion of cleaved versican, activating the epidermal growth factor receptor (EGFR), promoting invasion, and conferring anoikis resistance in renal cell carcinoma (RCC) cells. |
|
Tissue inhibitor of metalloproteinase 3 (TIMP3) expression reverses ADAMTS1-induced versican cleavage, epidermal growth factor receptor (EGFR) activation, and invasion and anoikis resistance of renal cell carcinoma (RCC) cells. |
|
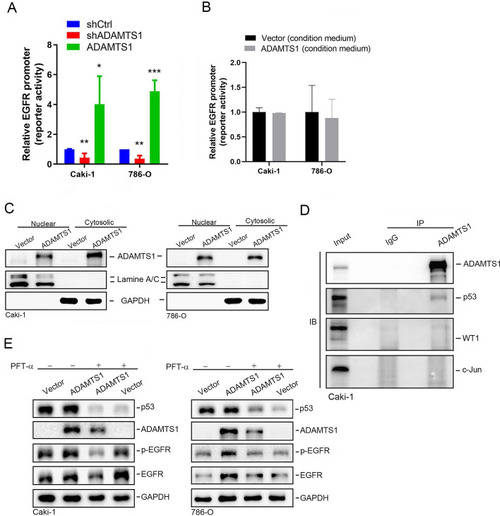
ADAMTS1 is associated with p53 to modulate epidermal growth factor receptor (EGFR) promoter activity and expression in renal cell carcinoma (RCC) cells. |
|
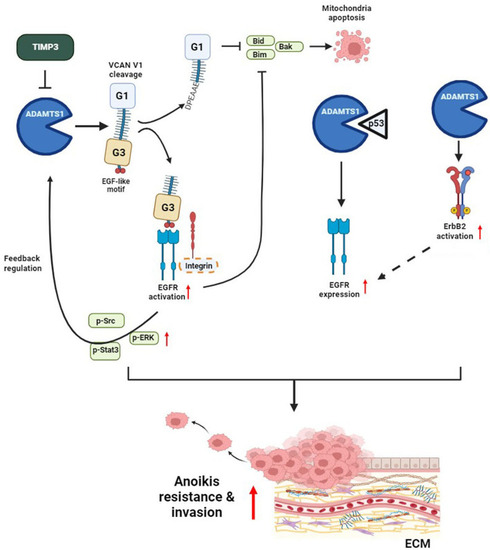
Schematic presentation depicting the ADAMTS1–VCAN–EGFR axis in promoting the anoikis resistance and invasive abilities of renal cell carcinoma (RCC). ADAMTS1 mediates the proteolytic cleavage of VCAN V1, thereby activating EGFR cascades to promote anoikis resistance and invasion of RCC cells. Moreover, ADAMTS1 interacts with p53 to modulate EGFR expression. EGFR-driven signaling might potentially reinforce ADAMTS1 expression through positive feedback. Bold dashed oval and arrow indicate hypothetical molecules and pathways that participate in ADAMTS1-mediated EGFR activation or expression. The cyclic elevation of ADAMTS1 and EGFR exacerbates anoikis resistance and invasiveness in RCC cells |