- Title
-
Induction of seizures and initiation of epileptogenesis by pilocarpine in zebrafish larvae
- Authors
- Gawel, K., Hulas-Stasiak, M., Marszalek-Grabska, M., Grenda, A., Siekierska, A., Kosheva, N., van der Ent, W., Esguerra, C.V., Krawczyk, P., Turski, W.A.
- Source
- Full text @ Front. Mol. Neurosci.
|
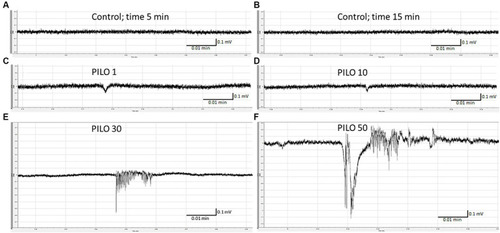
Local field potentials (LFPs) recorded from the brain of zebrafish larvae exposed to pilocarpine (PILO) at different concentrations. Larvae were incubated in different concentrations of PILO (1, 10, 30 or 50 mM) for 5 min, and, afterwards, LFPs were recorded for 20 min: |
|
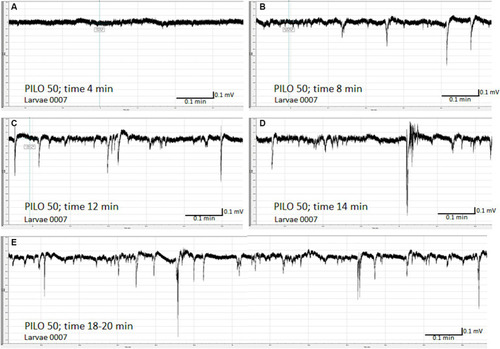
Time-dependent evolution of electroencephalographic changes recorded from the brain of zebrafish larvae exposed to pilocarpine (PILO). Larvae was incubated in medium containing PILO (50 mM) for 5 min, and, afterwards, LFPs were recorded for 20 min: |
|
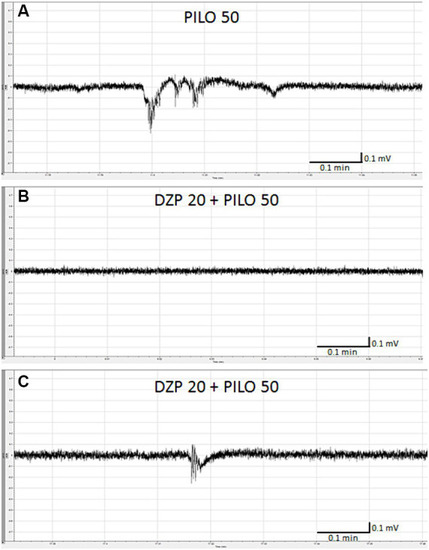
Effect of diazepam (DZP) on PILO-induced local field potential (LFP) events. Larvae were incubated in medium containing DZP (20 μM) for 20 h, and, subsequently, PILO was added to wells (final concentration = 50 mM). After 5 min incubation with PILO, LFPs recording were performed: |
|
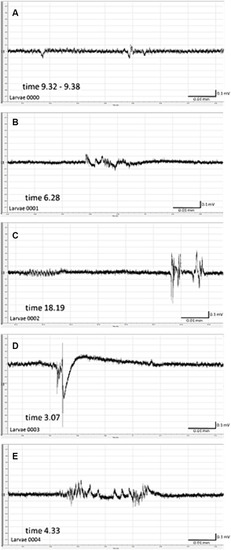
Spontaneous local field potentials (LFPs) recorded from the brain of zebrafish larvae previously exposed to pilocarpine (PILO). Larvae were incubated in medium containing PILO (50 mM) for 120 min, and, afterwards, larvae were thoroughly washed out 5 times in fresh medium and incubated in PILO-free medium for the next 22 h. Subsequently, LFP recordings were performed: |
|
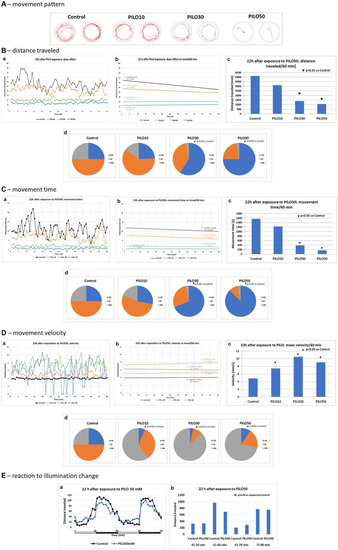
Locomotor activity of zebrafish larvae acutely exposed to pilocarpine (PILO). Larvae were incubated in different concentrations of PILO (1, 10, 30 or 50 mM) for 5 min and afterward locomotor activity assay was conducted for 60 min: |
|
Locomotor activity of zebrafish larvae 22 h after exposition to pilocarpine (PILO). Larvae were incubated in different concentrations of PILO (10, 30 or 50 mM) for 120 min. Next, larvae were thoroughly washed out 5 times, and incubated in fresh medium for subsequent 22 h. Afterwards, locomotor activity assay was conducted for 120 min: |
|
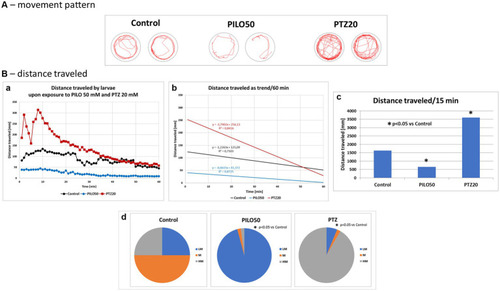
Comparison of locomotor activity of zebrafish larvae exposed to pilocarpine (PILO) or pentylenetetrazole (PTZ). Larvae were incubated in medium containing PILO (50 mM) or PTZ (20 mM) for 5 min, and, afterwards, locomotor activity assay was conducted for 60 min at 1 min intervals: |
|
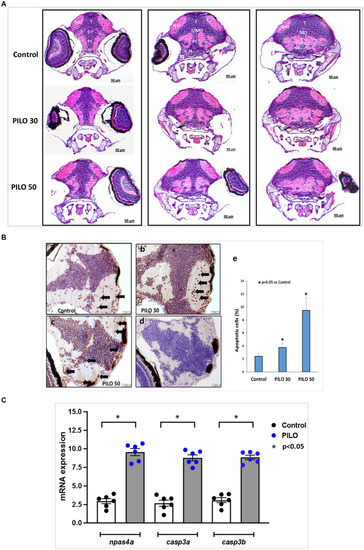
Histological changes in the brain of zebrafish larvae exposed to pilocarpine (PILO). |