- Title
-
Skeletal muscle regeneration after extensive cryoinjury of caudal myomeres in adult zebrafish
- Authors
- Oudhoff, H., Hisler, V., Baumgartner, F., Rees, L., Grepper, D., Jaźwińska, A.
- Source
- Full text @ NPJ Regen Med
|
Myomere organization in adult zebrafish, and the effects of cryoinjury of the caudal peduncle. |
|
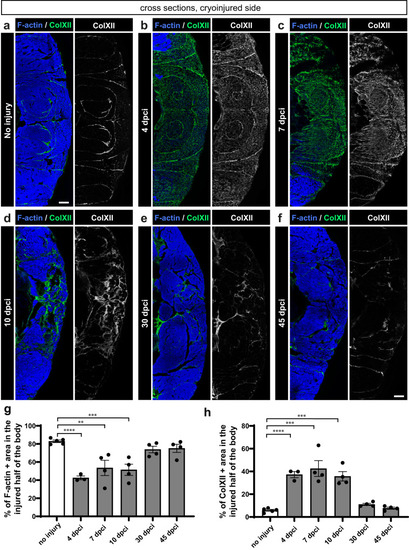
Dynamics of F-actin and Collagen XII in the wounded side of the fish body. |
|
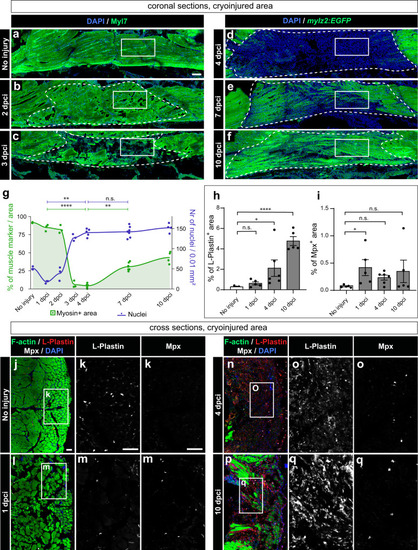
Muscle clearance is associated with cell infiltration and an immune response. |
|
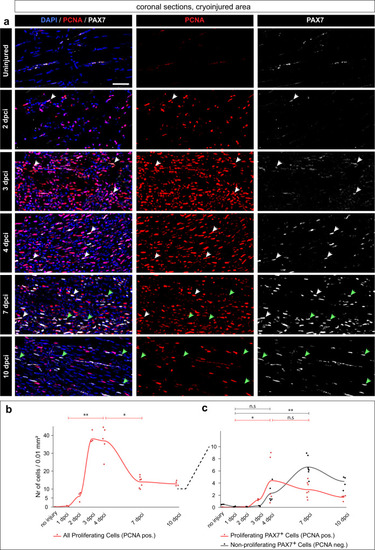
An increase of PAX7+ satellite cells and cell proliferation in the wound after muscle degeneration. |
|
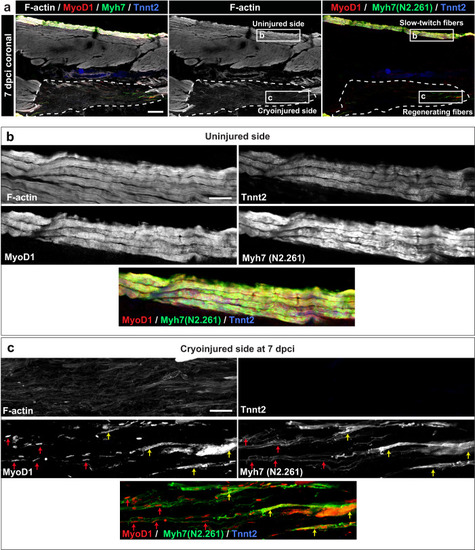
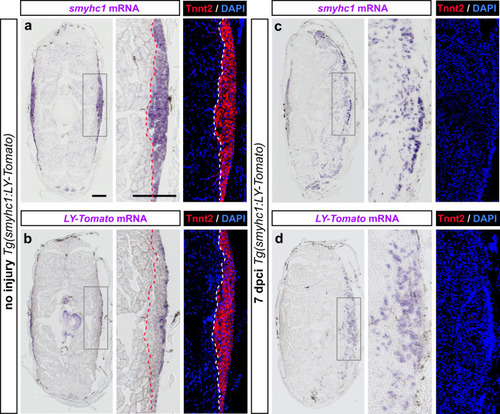
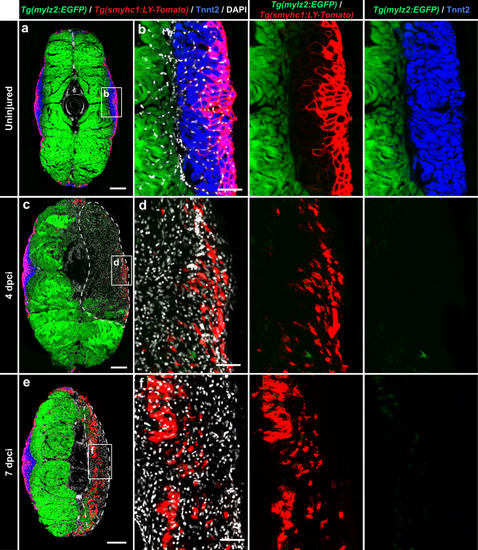
Expression of MyoD1 and slow muscle markers in early regenerating myofibers. |
|
Comparison between Transversal sections of |
|
The |
|
A nearly perfect restoration of fast and slow myofibers is accomplished at 30 to 45 dpci. Higher magnifications of cross sections displaying the injured side of |
|
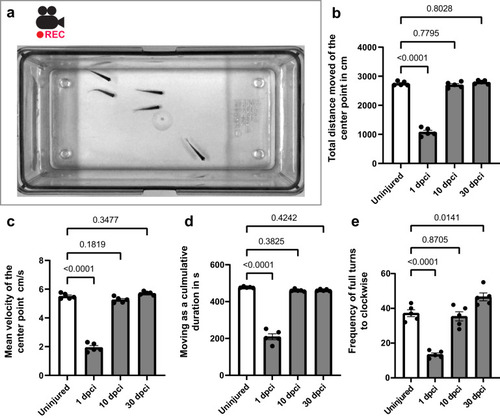
Zebrafish retain swimming activity after cryoinjury. |
|
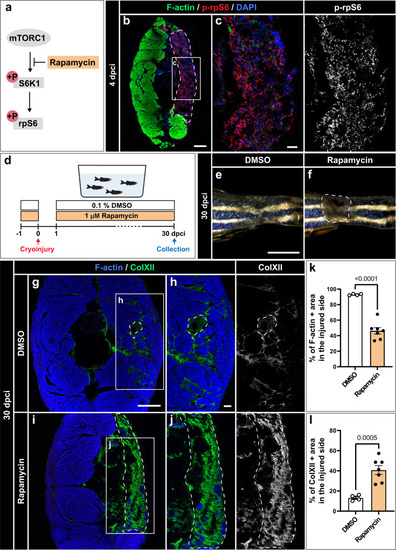
TOR signaling is required for muscle regeneration. |