- Title
-
Development and repair of blood vessels in the zebrafish spinal cord
- Authors
- Ribeiro, A., Rebocho da Costa, M., de Sena-Tomás, C., Rodrigues, E.C., Quitéria, R., Maçarico, T., Rosa Santos, S.C., Saúde, L.
- Source
- Full text @ Open Biol.
|
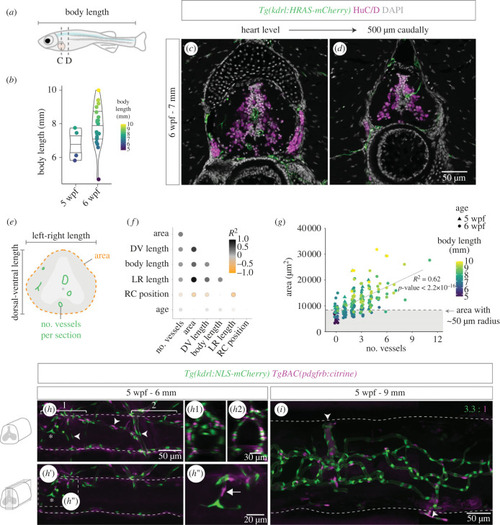
Spinal cord vascularization in juvenile zebrafish. ( |
|
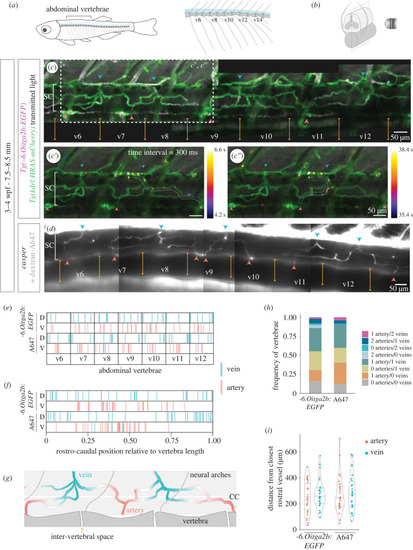
Direction of blood flow and distribution of arteries and veins in the developing spinal cord vasculature. ( |
|
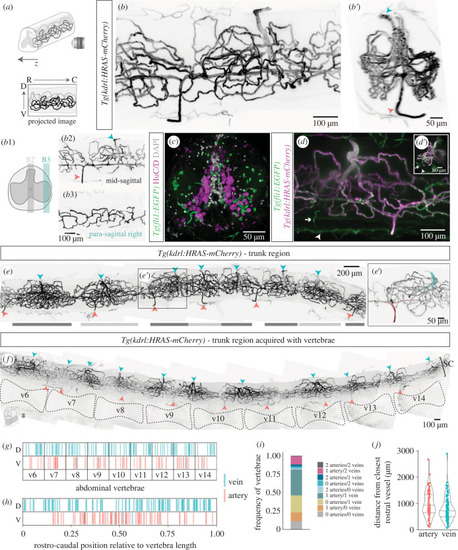
Organization of the spinal cord vasculature in adult zebrafish. ( |
|
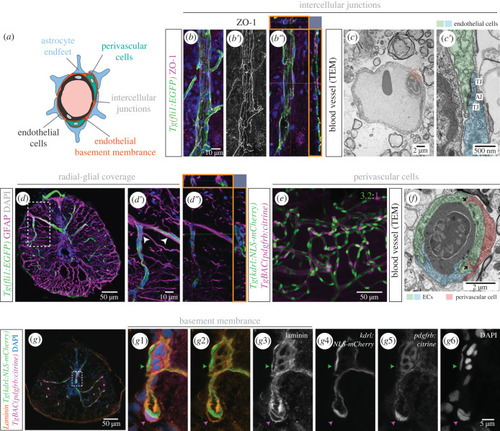
Organization of the blood-spinal cord barrier in adult zebrafish. ( |
|
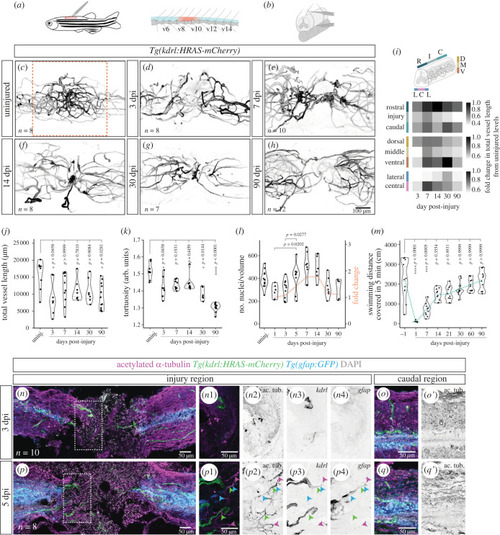
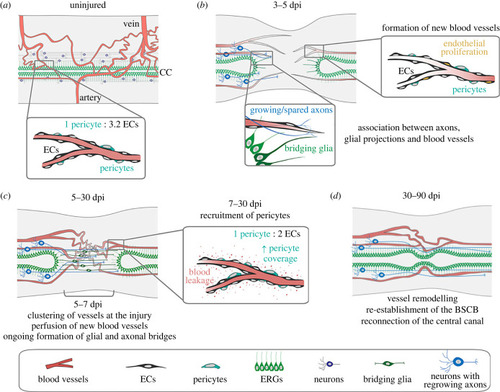
Changes in vascular distribution and morphology in response to spinal cord injury. ( |
|
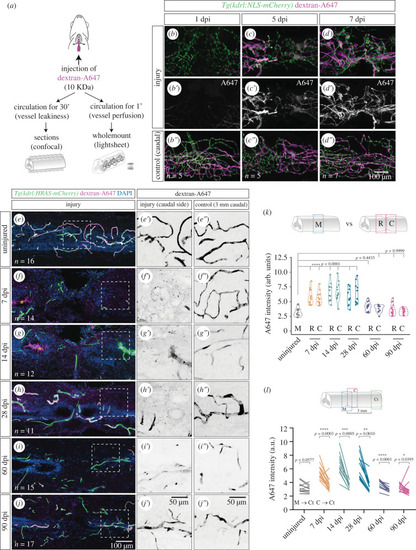
Re-establishment of the blood-spinal cord barrier after injury. ( |
|
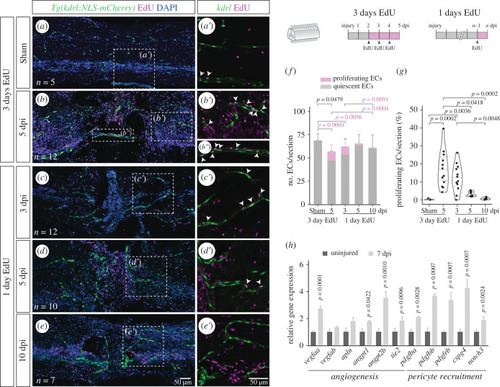
Endothelial proliferation during vascular repair. ( |
|
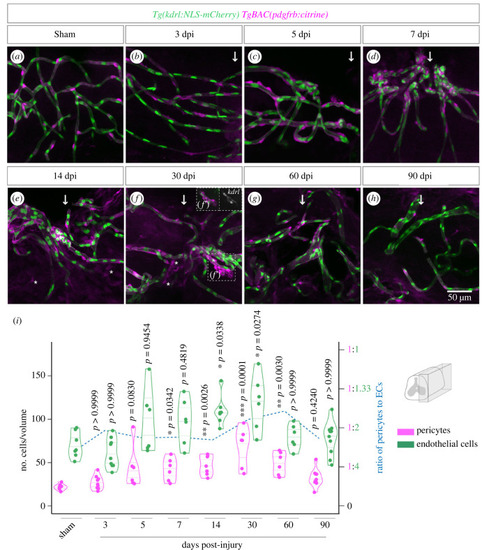
Pericyte recruitment during vascular repair. ( |
|
Model of vascular repair in the zebrafish spinal cord. ( |