- Title
-
Chemokine signaling synchronizes angioblast proliferation and differentiation during pharyngeal arch artery vasculogenesis
- Authors
- Liu, J., Zhang, M., Dong, H., Liu, J., Mao, A., Ning, G., Cao, Y., Zhang, Y., Wang, Q.
- Source
- Full text @ Development
|
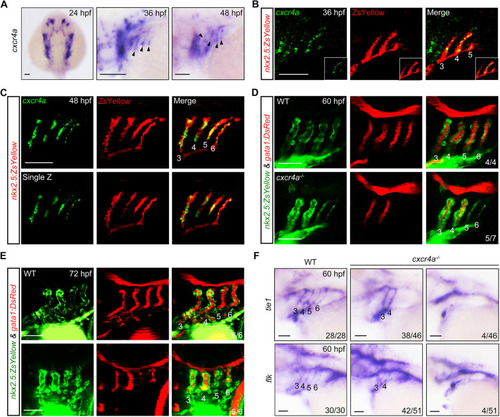
Genetic depletion of cxcr4a induces PAA malformation. (A) The expression of cxcr4a at 24 (dorsal view with anterior towards the top), 36 and 48 hpf (lateral views with anterior towards the left). Black arrowheads indicate the expression of cxcr4a in the pharynx. (B,C) Colocalization of cxcr4a transcripts and ZsYellow+ PAA angioblasts. Tg(nkx2.5:ZsYellow) embryos were stained with anti-ZsYellow antibody (red) and hybridized with cxcr4a probes (green) by fluorescent in situ hybridization. (D,E) Confocal images depicting blood flows in wild-type (WT) and cxcr4a-deficient embryos. PAAs 3-6 are marked with corresponding numbers. The ratios of affected embryos are indicated in the lower right corner. (F) The expression of tie1 and flk in wild type (WT) and cxcr4a−/− mutants. Scale bars: 50 μm. |
|
Lack of cxcr4a represses both the proliferation and differentiation of PAA angioblasts. (A) Time-lapse recording of ZsYellow fluorescence in the pharyngeal regions of wild-type (WT) and cxcr4a−/− embryos in Tg(nkx2.5:ZsYellow) background. (B-D) cxcr4a−/− mutants showed a significantly reduced cell number in posterior PAAs. Representative confocal sections of the PAAs in wild-type (WT) control and cxcr4a-deficient embryos are shown in B. Quantified cell numbers in PAAs 3-6 are shown in C and D. Error bars indicate s.d. of three biological replicates. (E,F) Detection of proliferative PAA angioblasts. Wild-type (WT) and cxcr4a−/− embryos were co-immunostained using anti-ZsYellow (green) and anti-BrdU (red) antibodies. Nuclear DNA was stained using DAPI (blue). Representative pictures are shown in E and the percentage of BrdU-positive cells in PAAs 5 and 6 for each group is shown in F. Error bars indicate s.d. of three biological replicates. (G,H) Expression analysis of tie1 in the wild-type (WT) and cxcr4a−/− embryos at 48 hpf by in situ hybridization (G) and fluorescent in situ hybridization combined with immunostaining (H). (I) Cdh5 levels were greatly decreased in the posterior PAAs of cxcr4a−/− mutants. The indicated embryos were immunostained using Cdh5 (green) and ZsYellow (red) antibodies. Scale bars: 50 μm. |
|
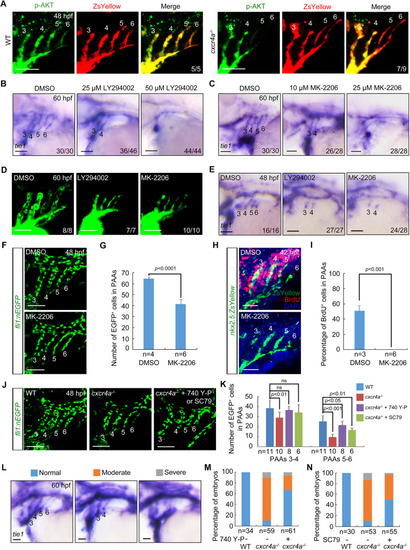
Cxcr4a regulates PAA development through the PI3K/AKT cascade. (A) AKT phosphorylation levels were decreased in PAAs 5 and 6 of cxcr4a−/− mutants. Control and cxcr4a−/−;Tg(nkx2.5:ZsYellow) embryos were immunostained using anti-p-AKT (green) and anti-ZsYellow (red) antibodies. (B,C) Wild-type embryos were treated with the PI3K inhibitor LY294002 (B) or the AKT inhibitor MK-2206 (C) from 18 hpf. Embryos were then harvested for in situ hybridization. (D) Live confocal images of Tg(nkx2.5:ZsYellow) embryos with 25 μM LY294002 or 10 μM MK-2206 from 18 to 60 hpf. (E) Embryos were treated with 25 μM LY294002 or 10 μM MK-2206 from 18 hpf until harvest for in situ hybridization. (F,G) Quantitative analysis of the number of PAA cells in embryos treated with either DMSO or 10 μM MK-2206. Representative pictures are shown in F and the number of PAA cells for each group is shown in G. Error bars indicate the s.d. of three biological replicates. (H,I) Confocal images of embryos treated with DMSO or 10 μM MK-2206 from 18 to 48 hpf. PAA angioblasts were labeled using antibody against ZsYellow (green), and proliferating cells were visualized by BrdU immunofluorescence (red) (H). Nuclei were stained with DAPI (blue). Ratios of BrdU-positive PAA cells were calculated and are shown in I. Error bars indicate the s.d. of three biological replicates. (J,K) Reduced cell number could be partially rescued by PI3K/AKT re-activation. cxcr4a−/− mutants were exposed to the PI3K agonist 740 Y-P (1 μM) or the AKT agonist SC79 (0.5 μM) from 18 to 48 hpf. Representative images are shown in J and quantified cell numbers are shown in K. Error bars indicate the s.d. of three biological replicates. Unpaired Student's t-test; ns, not significant. (L-N) cxcr4a−/− mutants were treated with 740 Y-P or SC79 from 18 to 60 hpf. Different phenotypes of PAAs were visualized using tie1 expression (L). The percentages of affected embryos are shown in M,N. Scale bars: 50 μm. |
|
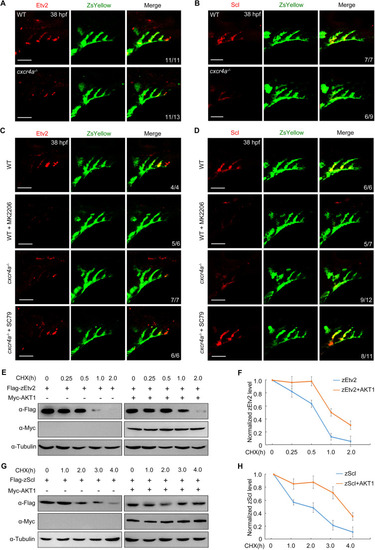
AKT is required for stabilization of zEtv2 and zScl. (A,B) Protein levels of zEtv2 and zScl were decreased in PAA clusters of cxcr4a−/− mutants. (C,D) Expression analysis of zEtv2 and zScl in wild type (WT) and cxcr4a−/− mutants on the Tg(nkx2.5:ZsYellow) background treated with 10 μM MK-2206 or 0.5 μM SC79 from 18 to 38 hpf. (E-H) HEK293T cells were transfected with indicated plasmids. (E,G) 24 h later, cells were treated with CHX (20 μg/ml) for the indicated times and harvested for immunoblotting. (F,H) Protein levels were quantified and normalized to tubulin (mean±s.d., three independent biological replicates). Scale bars: 50 μm. |
|
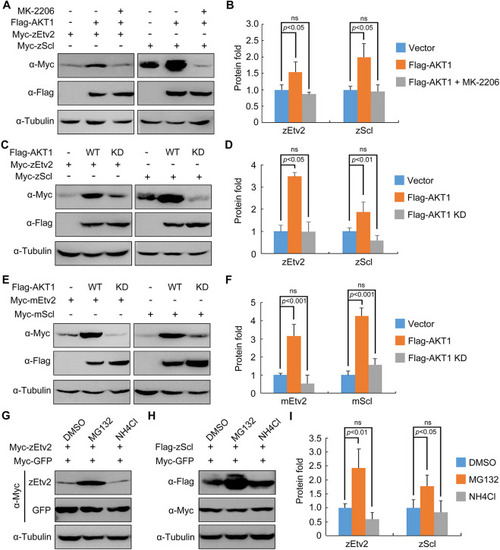
AKT stabilizes zEtv2 and zScl through its kinase activity. (A,B) HEK293T cells were co-transfected with plasmids expressing Flag-AKT1 and Myc-zEtv2 or -zScl, and treated with either DMSO or 0.5 μM MK-2206 for 24 h before undergoing western blot analysis. (C,D) Overexpression of wild-type (WT) AKT1 but not its kinase-deficient (KD) mutant stabilizes zEtv2 and zScl. (E,F) The promotion of mEtv2 or mScl expression level by AKT1 is dependent on its kinase activity. (G-I) zEtv2 and zScl proteins are degraded through the proteasome pathway. HEK293T cells co-transfected with plasmids encoding either Myc-zEtv2 or Flag-zScl and the negative control Myc-GFP were treated with either the lysozyme inhibitor NH4Cl (20 mM) or the proteasomal inhibitor MG132 (20 μM) for 5 h before harvest. In B,D,F,I, the gray values of the immunoreactive protein bands were quantified using ImageJ. Graphs show the density of the indicated protein signals relative to the corresponding Tubulin signals (mean±s.d., three independent biological repeats). Paired Student's t-test; ns, not significant. |
|
AKT1 directly interacts with zEtv2 and zScl. (A,B) AKT1 interacts with zEtv2 and zScl. HEK293T cells transfected with the indicated plasmids were harvested for immunoprecipitation with anti-Flag antibody and western blot analyses. (C) Overexpressed AKT1 interacts with endogenous zEtv2 and zScl. Wild-type embryos were injected with 200 pg Flag-AKT1 mRNA at the one-cell stage, and then harvested for immunoprecipitation with anti-Flag antibody. (D,E) Direct binding of AKT1 to zEtv2 or zScl in vitro. GST, GST-AKT1, GST-zEtv2 (236-345) and GST-zScl were expressed in bacterial cells and purified. The purified GST-fusion proteins GST-zEtv2 (236-345) and GST-zScl were then treated with thrombin to cleave their GST tags, after which they were incubated with GST-AKT1. The pull-down fraction was separated with SDS-PAGE and analyzed by Coomassie Blue staining. |
|
AKT phosphorylates zEtv2 at S296 and zScl at S222. (A-C) AKT increases the phospho-serine levels of zEtv2 and zScl. HEK293T cells were transfected with the indicated plasmids and treated with 20 μM MG132 for 5 h before harvest. Lysates were immunoprecipitated with anti-p-serine antibody and blotted with anti-Flag antibody. (D-F) The expression of wild-type zEtv2 and zScl – but not their phosphorylation-resistant mutants – was promoted by AKT1. (G,H) In vitro kinase assays revealed that AKT phosphorylated wild-type zEtv2 and zScl, but not their phosphorylation-resistant mutants. Flag-AKT1 was expressed in HEK293T cells and immunoprecipitated using anti-Flag-agarose beads. Wild-type and mutant proteins were purified from bacterial cells. The AKT-mediated phosphorylation of these purified proteins was detected by western blots using anti-p-serine antibody. (I-K) The phospho-mimicking mutants for zEtv2 and zScl exhibited greater stability than their wild-type proteins. HEK293T cells were transfected with the indicated plasmids and then harvested for immunoblotting. The zEtv2-S296D and zScl-S222D mutants were more stable than their corresponding wild-type forms, and were not further stabilized by AKT1. The gray values were quantified using ImageJ for quantification of phosphorylation levels (C) or protein expression levels (F,K) of zEtv2 and zScl (mean±s.d., three independent biological repeats). Paired Student's t-test; ns, not significant. |
|
AKT suppresses the polyubiquitylation of zEtv2 and zScl. (A) zEtv2 and zScl degrade through the ubiquitin-proteasome pathway. Flag-tagged β-catenin, zEtv2 and zScl were co-expressed with Ub-K48R/G76A, a dominant-negative form of ubiquitin, in HEK293T cells. Lysates were then immunoblotted with the indicated antibodies. (B,C) AKT1 phosphorylates zEtv2 and zScl on specific serine residues to suppress their polyubiquitylation. HEK293T cells were transfected with the indicated constructs and incubated with 20 μM MG132 for 5 h before lysis. Ubiquitylated proteins were isolated by immunoprecipitation and ubiquitylation signals were detected by immunoblotting with an anti-HA antibody. Overexpression of wild-type (WT) AKT1, but not its kinase-deficient (KD) mutant, reduced the polyubiquitin modifications in zEtv2 and zScl proteins (B). Meanwhile, ectopic expression of AKT1 had a much more evident effect on the polyubiquitylation of the phosphorylation-resistant mutants of zEtv2 and zScl (zEtv2-S296A and zScl-S222A) compared with that of the relevant wild-type (WT) proteins (C). (D-F) Overexpression of either zEtv2/zScl-WT or their S-D form proteins in cxcr4a−/− mutants partially rescued tie1 expression in PAAs 5 and 6. Wild-type (WT) or cxcr4a−/− mutant embryos were injected with 200 pg of zEtv2/zScl-WT or their S-D or S-A form mRNA and AS-Flag-photo-MO at the one-cell stage. After treatment with 365 nm UV for 10 min at 30 hpf, the injected mRNA was overexpressed in the embryos along with AS-Flag-photo-MO degradation, and embryos were collected at 48 hpf for in situ hybridization (D). Scale bars: 50 μm. The percentages of embryos with defects in tie1 expression are shown in E,F. |
|
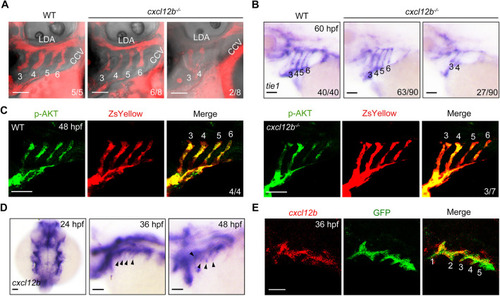
cxcl12b is expressed in pharyngeal pouches and is required for PAA morphogenesis. (A) Deletion of cxcl12b led to an interruption of blood flow in the posterior PAAs. Wild-type (WT) embryos and cxcl12b−/− mutants were injected with rhodamine-dextran into the common cardinal vein at 72 hpf. The blood flow in PAAs was imaged using a Nikon A1R+ confocal microscope. (B) Analysis of tie1 expression in wild-type (WT) embryos and cxcl12b−/− mutants. (C) Wild-type (WT) and cxcl12b−/− embryos on Tg(nkx2.5:ZsYellow) background were immunostained using anti-p-AKT (green) and anti-ZsYellow (red) antibodies. (D) Expression analysis of cxcl12b in wild-type embryos during PAA development. Black arrowheads indicate the expression of cxcl12b in the pharynx. (E) Colocalization analysis of cxcl12b transcripts with GFP proteins in pharyngeal pouches. Tg(sox17:GFP) transgenic embryos stained using anti-GFP (green) antibody were hybridized with cxcl12b probe (red). Scale bars: 50 μm. |