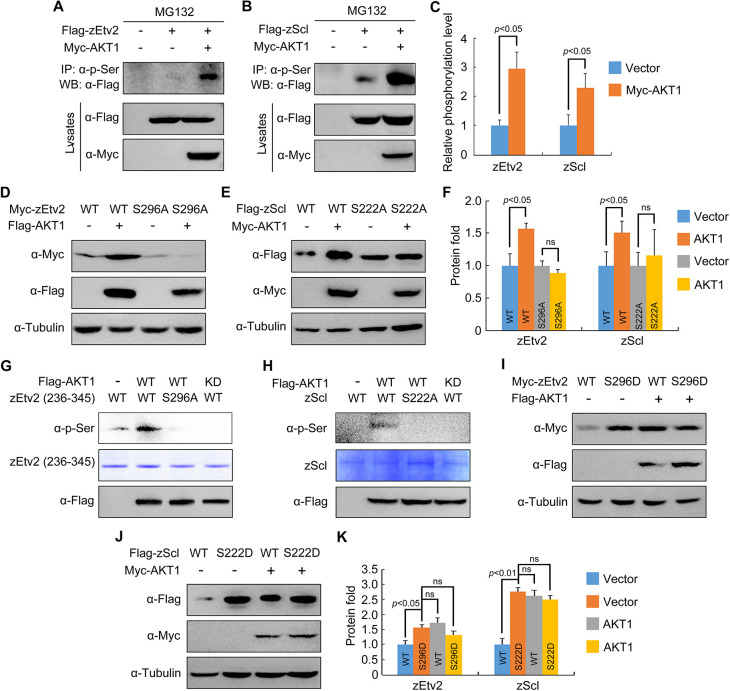
Fig. 7.
AKT phosphorylates zEtv2 at S296 and zScl at S222. (A-C) AKT increases the phospho-serine levels of zEtv2 and zScl. HEK293T cells were transfected with the indicated plasmids and treated with 20 μM MG132 for 5 h before harvest. Lysates were immunoprecipitated with anti-p-serine antibody and blotted with anti-Flag antibody. (D-F) The expression of wild-type zEtv2 and zScl – but not their phosphorylation-resistant mutants – was promoted by AKT1. (G,H) In vitro kinase assays revealed that AKT phosphorylated wild-type zEtv2 and zScl, but not their phosphorylation-resistant mutants. Flag-AKT1 was expressed in HEK293T cells and immunoprecipitated using anti-Flag-agarose beads. Wild-type and mutant proteins were purified from bacterial cells. The AKT-mediated phosphorylation of these purified proteins was detected by western blots using anti-p-serine antibody. (I-K) The phospho-mimicking mutants for zEtv2 and zScl exhibited greater stability than their wild-type proteins. HEK293T cells were transfected with the indicated plasmids and then harvested for immunoblotting. The zEtv2-S296D and zScl-S222D mutants were more stable than their corresponding wild-type forms, and were not further stabilized by AKT1. The gray values were quantified using ImageJ for quantification of phosphorylation levels (C) or protein expression levels (F,K) of zEtv2 and zScl (mean±s.d., three independent biological repeats). Paired Student's t-test; ns, not significant.