- Title
-
Histone Deacetylases Cooperate with NF-κB to Support the Immediate Migratory Response after Zebrafish Pronephros Injury
- Authors
- Zhuang, M., Scholz, A., Walz, G., Yakulov, T.A.
- Source
- Full text @ Int. J. Mol. Sci.
|
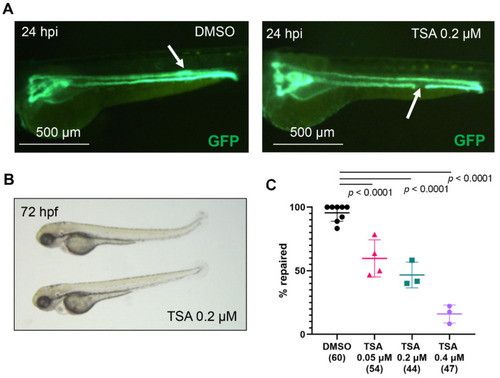
Histone deacetylases are required for zebrafish pronephros repair after injury. (A) The pronephros in a control, DMSO-treated embryo of the transgenic Tg(wt1b:GFP; cdh17:GFP) zebrafish line is fully repaired at 24 h post injury (hpi, left panel). The white arrow points to the location of the repaired tubule, which is characterized by a typical thickening of the duct. In contrast, a TSA-treated embryo fails to repair the pronephros (right panel). The arrow points to the ablated portion of the tubule. (B) Zebrafish embryos, incubated in 0.2 µM TSA for one hour before ablation and 24 h post ablation developed normally. (C) TSA treatment significantly reduced the number of embryos that repaired the tubule after laser-induced injury in a concentration-dependent manner. The concentration (micromolar, µM) is shown below each group. The number of examined embryos is displayed in brackets. Individual experiments are plotted, and the mean and standard deviation for each group are displayed. Significance was calculated using Fisher’s exact test (two-tailed). PHENOTYPE:
|
|
Hdac8 and Hdac2 activities are required for pronephros repair after laser-induced injury. (A) Embryos, incubated in 1 µM PCI-34051 (upper panel) or 10 µM PCI-34051 (lower panel) from 48 to 72 hpf developed normally. (B) PCI-34051 affects the pronephros repair in a concentration-dependent manner. Embryos were incubated in DMSO or increasing concentrations of the specific Hdac8 inhibitor PCI-34051 for one hour before ablation and for 24 h post injury (hpi). (C) Treatment of zebrafish embryos from 48 to 72 hpf with 1 µM or 15 µM solution of the Hdac2 inhibitor CAY10683 did not affect development. (D) Treatment of zebrafish larvae with 15 µM solution of CAY10683 (10683) significantly increased the percentage of embryos that did not repair the gap after 24 h. The numbers of examined embryos are displayed in brackets. The PCI-34051 concentrations (micromolar, µM) are shown below each group. The points represent individual experiments. Mean and standard deviation for each group are displayed. Significance was calculated using Fisher’s exact test (two-tailed). |
|
Hdac6 activity is required for zebrafish pronephros repair after injury. (A) Development was not affected in embryos incubated in 1 µM (upper panel) or 5 µM (lower panel) solution of the Hdac6 inhibitor CAY10603. (B) Only 60% of the embryos treated with 1 µM solution of the Hdac6 inhibitor CAY10603 (10603) repaired the gap after laser-induced injury. The group size is shown in brackets (Fisher’s exact test, two-tailed). (C) The combined Hdac2, Hdac6, and Hdac8 activities are required for the migratory response after injury. Incubation of 48 h old zebrafish in solution containing CAY10603, PCI-34051 and CAY10683 (5 µM, upper panel; 10 µM, lower panel) did not affect embryonic development. (D) Less than half of the embryos incubated in solution containing CAY10603, PCI-34051, and CAY10683 repaired the tubule after injury. The group sizes are shown in brackets below each group. Individual experiments with mean and standard deviation for each group are displayed. p-values were calculated with Fisher’s exact test (two-tailed). |
|
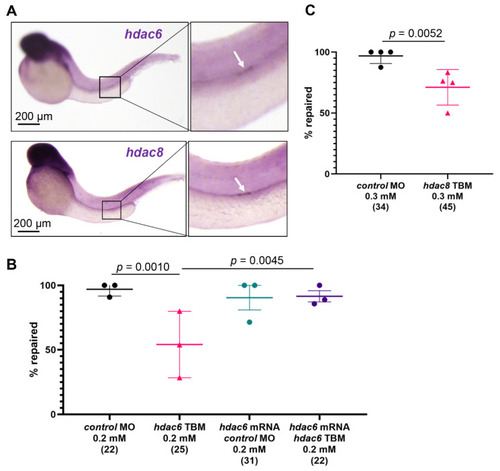
hdac6 and hdac8 are necessary for the pronephros repair process. (A) In situ hybridization revealed an upregulation of hdac6 (upper panel) and hdac8 (lower panel) two hours after laser-induced injury. The arrow points to the injury site. (B) Depletion of zebrafish hdac6 by translation- (TBM) blocking morpholino oligonucleotides (MO) significantly reduced the percentage of embryos that repaired the pronephric tubule (Fisher’s exact test, two-tailed). Co-injection of hdac6 mRNA rescued the hdac6 TBM pronephros repair phenotype. (C) Depletion of hdac8 with translation-blocking morpholino oligonucleotides significantly reduced number of repaired pronephri. The group size is shown in brackets. Individual experiments with mean and standard deviation for each group are displayed. Significance was calculated with Fisher’s exact test (two-tailed). EXPRESSION / LABELING:
PHENOTYPE:
|
|
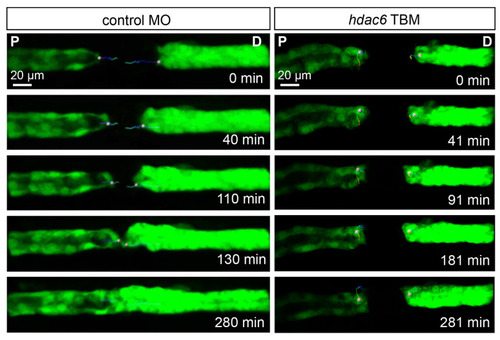
hdac6 depletion affects the migratory response after laser-induced injury. Frames from a time-lapse movies of control MO-injected and hdac6 TBM-injected embryos after an injury. While the control embryos closed the 80 µm gap within three hours, the cells flanking the injury in hdac6 TBM-injected embryos showed little to no migratory response (P: proximal; D: distal). The trajectories of one proximal and one distal leading cell per sample are depicted with color-coded lines (blue: early times; red: late times). PHENOTYPE:
|
|
Histone deacetylases and NF-κB signaling cooperate to promote repair through migration. (A) Whole-mount in situ hybridization reveals that nfkbiaa is upregulated in the cells flanking the injury site and the expression is persistent in the first 4 h post injury (hpi). (B) An example of nfkbiaa SBM-injected embryo 24 h post ablation. The white arrow points to the injury site. (C) Depletion of nfkbiaa with splice blocking MO (SBM) significantly reduced the number of successful repairs. (D) nfkbiaa TBM-injected embryos repair at significantly lower rate than controls. (E) Combined depletion of hdac6 TBM, hdac8 TBM, and nfkbiaa TBM with low MO concentrations synergistically reduces the number of embryos that successfully repair the pronephros. The number of examined embryos is shown in brackets. The points represent individual experiments. Mean and standard deviation for each group are displayed. Significance was calculated with Fisher’s exact test (two-tailed). PHENOTYPE:
|