- Title
-
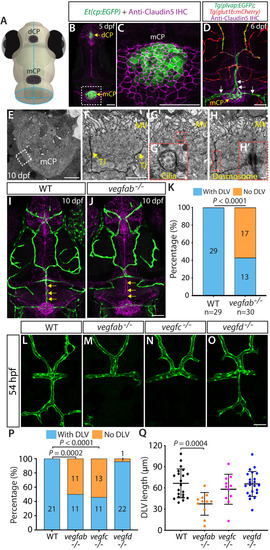
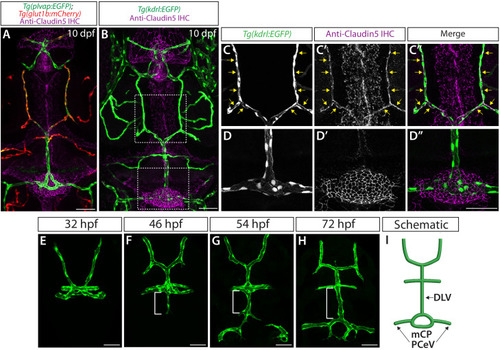
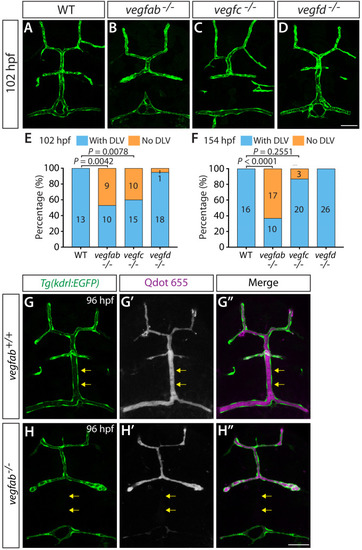
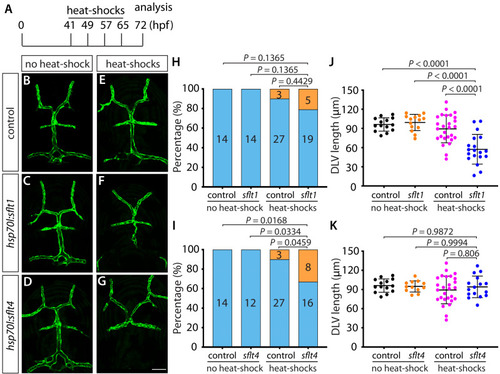
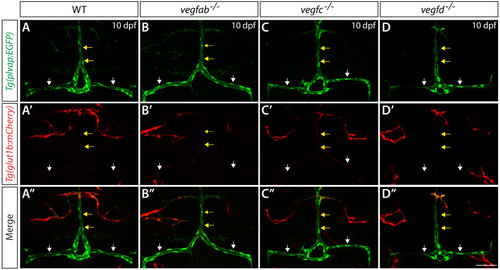
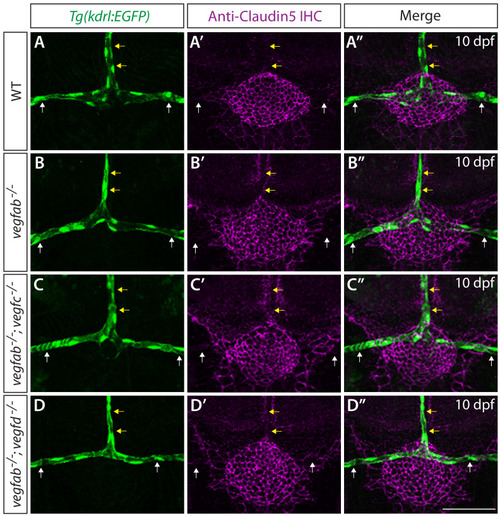
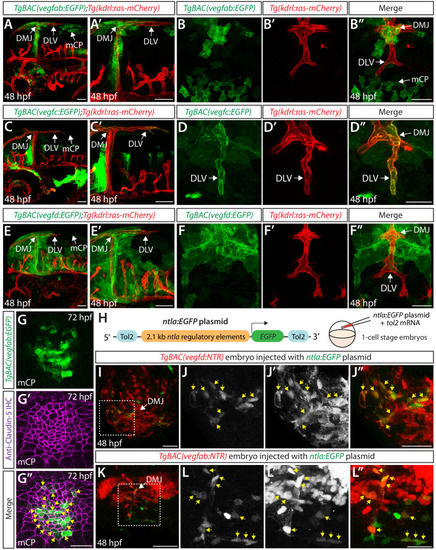
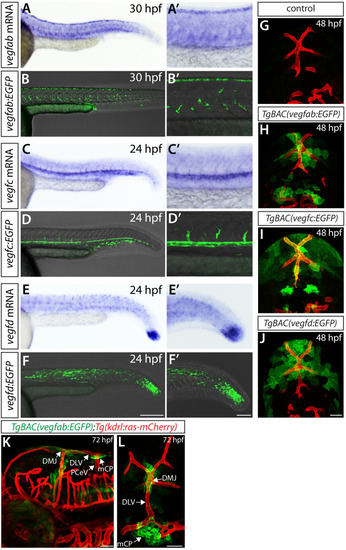
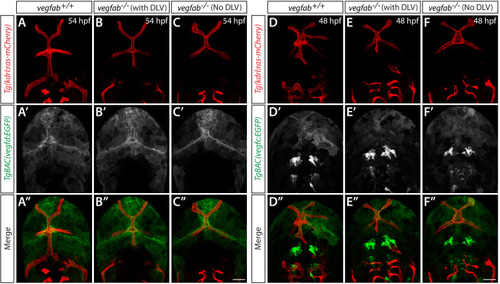
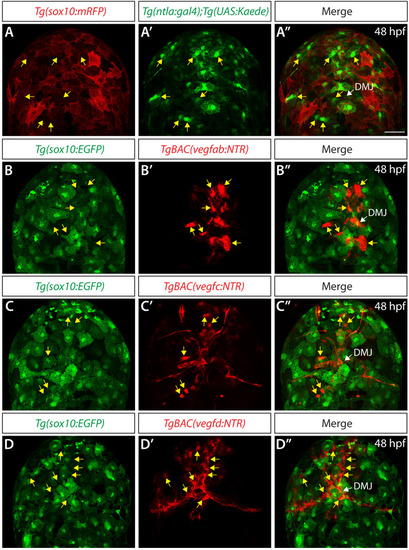
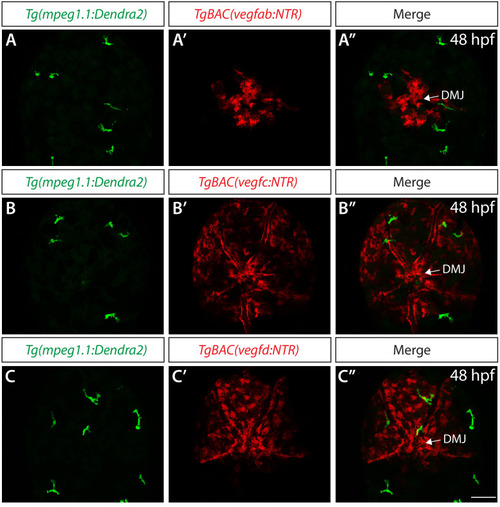
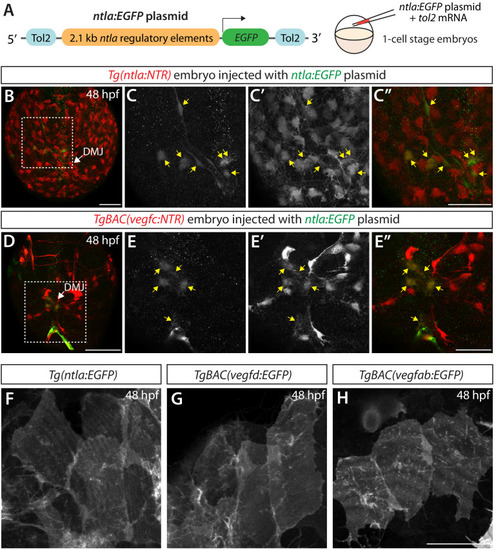
Endothelial cell type-specific molecular requirements for angiogenesis drive fenestrated vessel development in the brain
- Authors
- Parab, S., Quick, R.E., Matsuoka, R.L.
- Source
- Full text @ Elife
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |