- Title
-
Berbamine Analogs Exhibit Differential Protective Effects From Aminoglycoside-Induced Hair Cell Death
- Authors
- Hudson, A.M., Lockard, G.M., Namjoshi, O.A., Wilson, J.W., Kindt, K.S., Blough, B.E., Coffin, A.B.
- Source
- Full text @ Front. Cell. Neurosci.
|
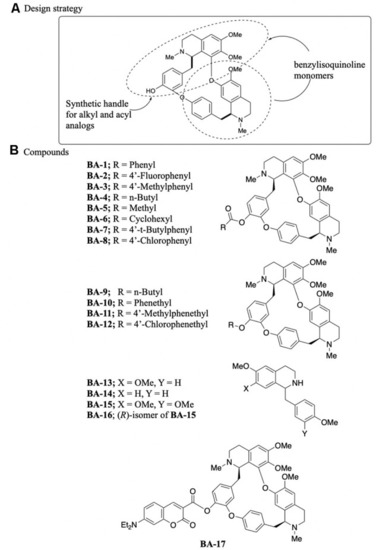
Synthesis of berbamine analogs. |
|
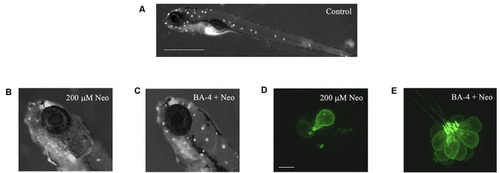
Berbamine analogs confer protection from neomycin. Analog BA-4 is shown here as an example. |
|
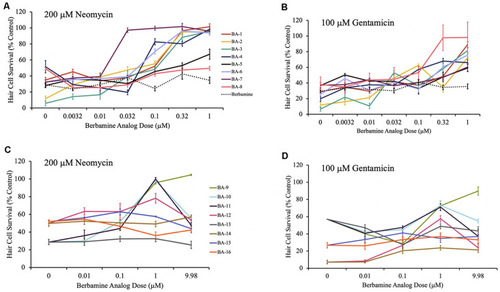
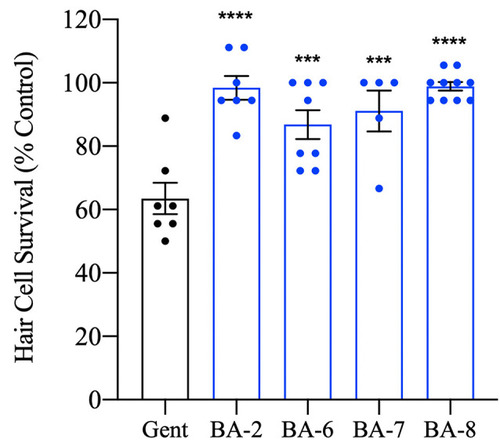
Berbamine analogs confer protection from aminoglycosides. |
|
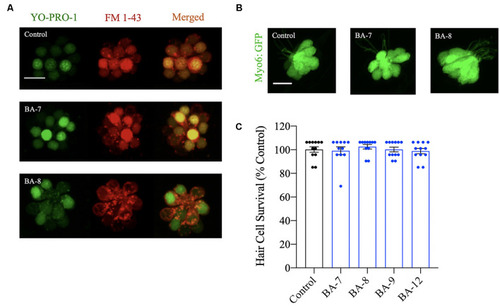
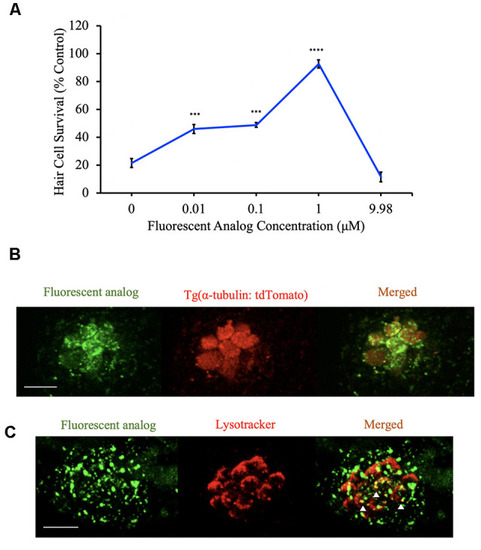
Hair cells are viable after analog exposure. |
|
Hair cell protection persists after 24 h. Zebrafish were pretreated with the OPC of analog for 1 h followed by 200 μM gentamicin treatment for 30 min. Fish were allowed to recover for 24 h before the assessment. All analogs tested significantly prevented hair cell death after 24 h suggesting true protection rather than a delay in cell death onset. Hair cells were assessed |
|
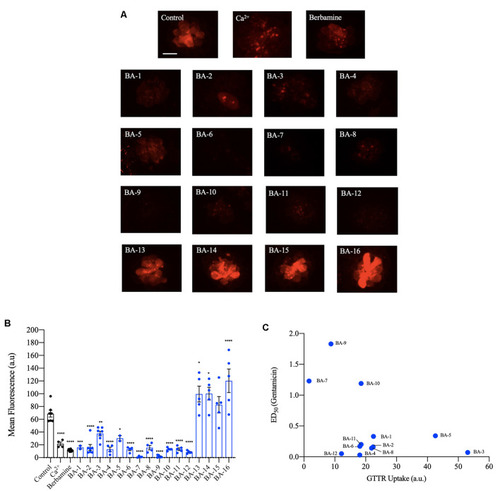
Berbamine analogs reduce aminoglycoside uptake by hair cells. |
|
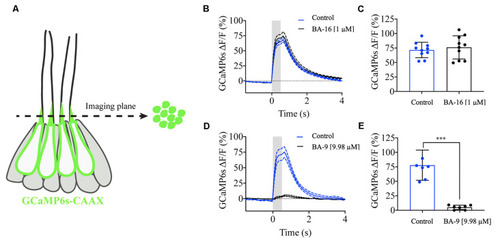
Berbamine analogs differentially reduce mechanotransduction channel activity. Mechanosensitive-calcium signals from hair bundles of posterior lateral line neuromasts. |
|
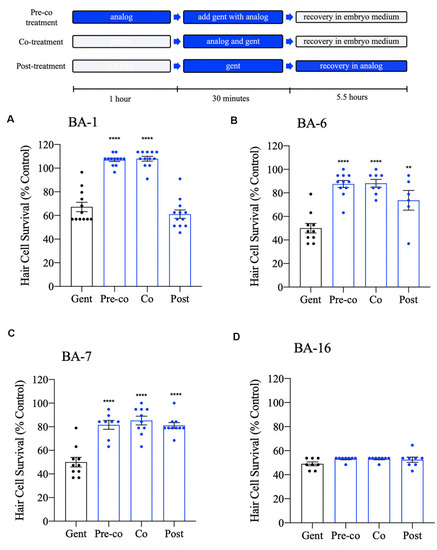
Differential patterns of analog protection from gentamicin. Zebrafish were either pre-treated with the OPC of analog for 1 h, then co-treated with analog and gentamicin for 30 min (“pre-co” condition), co-treated with analog and gentamicin without a pretreatment period (“co” condition) or post-treated with analog for 5.5 h following gentamicin removal (“post” condition). All treatments used 200 μM gentamicin. Examples are shown for analogs that represent each pattern of protection. |
|
Berbamine analogs enter hair cells. |
|
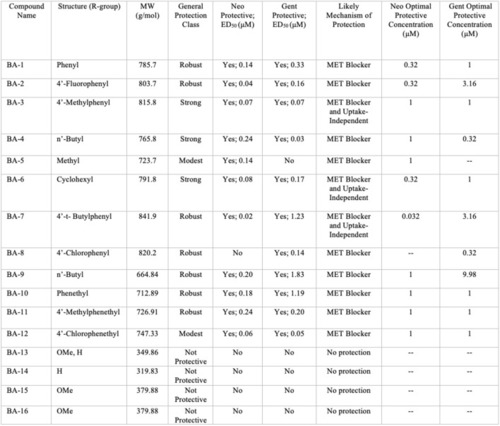
Summary of berbamine analog protection and compound characteristics. Compound name, structure, molecular weight, protection class, likely mechanism of protection, ED50, and OPCs against neomycin and gentamicin for all 16 berbamine analogs. The general protection classes were defined based on thresholds of protection levels by percent. Robust = 60% increase in hair cell protection compared to aminoglycoside group; Strong = over 40% increase in hair cell protection compared to aminoglycoside group; Modest = over 30% increase in hair cell protection compared to aminoglycoside group; No protection = less than 30% of hair cell protection compared to aminoglycoside group. The likely mechanism was determined by the GTTR experiments in |