- Title
-
FGF and canonical Wnt signaling cooperate to induce paraxial mesoderm from tailbud neuromesodermal progenitors through regulation of a two-step EMT
- Authors
- Goto, H., Kimmey, S.C., Row, R.H., Matus, D.Q., Martin, B.L.
- Source
- Full text @ Development
|
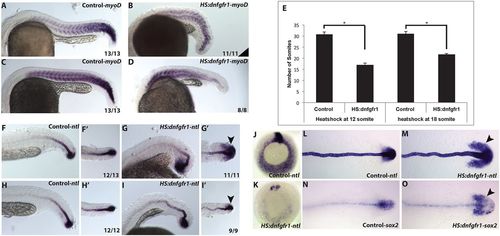
FGF is required for post-gastrula mesoderm induction and repression of the NMP state. (A-E) Zebrafish embryos from an HS:dnfgfr1 hemizygous outcross were heat shocked at the 12- or 18-somite stage and analyzed for myoD expression at 24 hpf. Wild-type siblings exhibit ∼31 somites (A,C,E), whereas HS:dnfgfr1 embryos heat shocked at 12 somites have 16 somites and those heat shocked at 18 somites have 22 somites (B,D,E). The number of embryos showing the illustrated phenotype among the total number examined is indicated. Error bars indicate s.d. *P<0.001 using unpaired t-test. (F-I') Expression of ntla (arrowheads) indicates that there is no posterior loss of notochord after FGF inhibition at the 12- or 18-somite stage. (J,K) Embryos heat shocked at 50% epiboly (just at the start of gastrulation) and fixed 3 h after heat shock show a near complete loss of ntla expression. (L,M) Embryos heat shocked at the 8-somite stage and fixed 3 h later exhibit an expansion of ntla into lateral and anterior domains (arrowhead). (N,O) Inhibition of FGF signaling at the 8-somite stage also leads to an expansion of sox2 3 h after the heat shock. (A-D,F,G,H,I) Lateral view, anterior left; (F′,G′,H′,I′,L-O) dorsal view, anterior left; (J,K) vegetal view, dorsal top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Two vimentin-related genes, vimr1 and vimr2, are expressed specifically in cells undergoing EMT in the tailbud. Expression pattern of vimr1 (A) and vimr2 (B) in wild-type 18-somite stage embryos. Expression of both genes is limited to the notochord and tailbud cells during somitogenesis (white arrowheads indicate notochord expression, black arrowheads tailbud expression). (C) Protein alignment derived from vimentin gene sequences. Red indicates a highly conserved motif, which has been shown to have a role in vimentin assembly (Herrmann et al., 1992); blue indicates the helix termination motif and purple indicates the beta site. Zebrafish sequences include that of another vimentin gene, vimentin-like (viml). (D) Bayesian phylogenetic analysis of Vimr1 and Vimr2 protein sequences with desmin proteins serving as an outgroup. Numbers at the node specify Bayesian posterior probabilities. Dr, Danio rerio (zebrafish); Hs, Homo sapiens (human); Mm, Mus musculus (mouse); On, Oreochromis niloticus (Nile tilapia); Sp, Stegastes partitus (bicolor damselfish); Tr, Takifugu rubripes (pufferfish); Xt, Xenopus tropicalis (frog). (E) Schematic of vimentin-related intermediate filament. Head and tail domains are indicated by dashed lines, whereas helix-rich regions of the rod domain are represented by filled boxes. (F-I) Expression of vimr1 and vimr2 in tbx16 (sptb104) mutants (H,I) and their wild-type control siblings (F,G). (A,B,F-I) Lateral views of the posterior tailbud domain. EXPRESSION / LABELING:
|
|
FGF is required for EMT termination during tailbud mesoderm formation. (A-H) The expression of vimr1 and vimr2 was examined at the 20-somite stage in HS:dnfgfr1 embryos (C,D) and their wild-type siblings (A,B) that were heat shocked at the 12-somite stage, as well as in HS:cafgfr1 embryos (G,H) and their wild-type siblings (E,F). Expression of vimr1 and vimr2 is increased in loss of FGF function embryos (C,D, arrowheads) and decreased in gain of FGF function embryos (G,H). (I-P′)The expression of tbx16 and msgn1 decreases in FGF-inhibited tailbuds (K-L′) as compared with sibling controls (I-J′). Conversely, expression of tbx16 and msgn1 increases in FGF-overactivation embryos (O-P′) compared with their wild-type controls (M-N′). White arrowheads (K′,L′; dorsal view) indicate loss of expression at the tip and midline of the tailbud. Black arrowheads (O′,P′; dorsal view) indicate ectopic expression at the tip and midline of the tailbud. (Q-R‴) Co-expression of vimr1 and Tbx16 was examined in 20-somite stage wild-type (Q-Q‴) and HS:dnfgfr1 (R-R‴) embryos that were heat shocked at 12 somites. FGF inhibition increases the expression of vimr1 while reducing the expression domain of Tbx16 (compare Q′,Q″ with R′,R″). All tailbud images are lateral views except where indicated. EXPRESSION / LABELING:
PHENOTYPE:
|
|
FGF regulates tailbud cell displacement through tbx16 regulation. (A-E) Time-lapse images of photoconverted tailbud cells of NLS-kikume mRNA-injected wild-type cells (Aa-d′,Ca-d′), HS:tbx16 (Ba-d′,Da-d′) and HS:cafgfr1 (Ea-d′) embryos at 12-somite stage and onward, as indicated. DMSO-treated wild-type cells were able to move rapidly from the progenitor zone (Aa-d′), whereas SU5402-treated wild-type cells remained within the tailbud (Ca-d′). Activation of tbx16 rescues cell displacement and speed in the presence of SU5402 (Da-d′). Cells with activated FGF signaling also enter the maturation zone similarly to wild-type embryos (Ea-d′ versus Aa-d′), but not as rapidly as tbx16-overexpressing cells (Ba-d′). White arrowheads point to cells entering the maturation zone. (F,G) Tracking of photoconverted cells as shown in trajectories (Fa-e) and displacement vector (Ga-e). (H-J) The mean displacement, mean track speed and mean track straightness of photoconverted cells. The mean displacement and the speed decrease in SU5402-treated wild-type embryos compared with vehicle-treated wild-type embryos (H versus I). tbx16 overexpression rescues the displacement and speed in SU5402-treated embryos. Track straightness did not differ significantly among the treatment groups (J). *P<0.05, unpaired two-way t-tests. PHENOTYPE:
|
|
FGF promotes mesoderm induction and inhibition of the NMP state via tbx16 and msgn1. (A-P) Expression of vimr1 and vimr2 was analyzed in SU5402- or DMSO-treated HS:tbx16, HS:msgn1 and wild-type sibling control embryos. Embryos were heat shocked at the 12-somite stage and analyzed at the 20-somite stage. Activation of tbx16 eliminates vimr1 and vimr2 expression in DMSO-treated (C,G) and SU5402-treated (compare D,H with B,F) embryos. Similarly, msgn1 activation inhibits vimr1/2 expression in DMSO-treated (K,O) and SU5402-treated (compare L,P with J,N) embryos. (Q-V) A transplant assay was used in epistasis experiments to determine whether tbx16 and msgn1 function downstream of FGF during termination and if these genes could rescue somite fate of FGF-inhibited tailbud cells. Wild-type donor embryos were injected with a mixture of fluorescein-dextran and heat shock-inducible HS:tbx16, HS:msgn1 or control HS:NLS-kikume plasmids. At sphere stage, the cells from these donor embryos were transplanted into the ventral margin of unlabeled wild-type shield stage host embryos, which targets them to the NMP population (Q). Embryos were heat shocked at the bud stage and examined at 30 hpf. The kikume protein was photoconverted prior to imaging and the lineage analysis was performed based on cell shapes and location of the transplanted cells. The majority of the transplanted wild-type cells contribute to somites, as do cells with tbx16 and msgn1 overexpression (R,V). The majority of the transplanted HS:dnfgfr1 cells are excluded from somites and do not form muscle (S-V) and remain in the tail region. However, upon the presence of tbx16 or msgn1, FGF-inhibited cells contribute to somites and form skeletal muscle (V). (W-AAf) A similar assay was used to determine whether transplanted cells that lack FGF function cell-autonomously maintain NMP markers sox2 and ntla (W). Host embryos containing transplanted wild-type cells and stained for sox2 (Xa-f) or ntla (Za-f) exhibit normal sox2 and ntla expression patterns, whereas host embryos with HS:dnfgfr1 cells have ectopic sox2 (Ya-f) and ntla (AAa-f) expressing cells in the tailbud (red arrowheads). Xa-e,Ya-e,Za-e,AAa-e, lateral views, anterior bottom; Xf,Yf,Zf,AAf, dorsal views, anterior left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Wnt promotes EMT initiation and cooperates with FGF to promote EMT termination in tailbud NMPs. (A-F) vimr1 and vimr2 expression was analyzed in 20-somite stage wild-type sibling (A,B), hemizygous HS:TCFΔC (C,D) and homozygous HS:TCFΔC (E,F) embryos. Wnt inhibition leads to the loss of vimr1 and vimr2. (G-J) Expression of vimr1 and vimr2 was also analyzed in HS:caβ-catenin embryos (I,J) and their wild-type siblings (G,H). Wnt overactivation leads to a loss of vimr1 and vimr2 expression and a shift towards the ventral anterior portion of the tailbud (black arrow in I,J). (K-T′) tbx16 and msgn1 expression was examined in control (K-L′,Q-R′), hemizygous HS:TCFΔC (M-N′), homozygous HS:TCFΔC (O-P′) and HS:caβ-catenin (S-T′) embryos. Wnt inhibition had little effect on tbx16 expression but led to decreased expression of msgn1. Wnt overactivation led to expansion of the tbx16 and msgn1 expression domains. (Ua-Xd) Epistasis experiments were performed to examine the control of vimr1 expression by the Wnt and FGF signaling pathways. HS:caβ-catenin embryos were heat shocked at the 12-somite stage and treated with SU5402 or vehicle only (DMSO). These embryos were subsequently fixed 3 h, 4 h, 5 h and 6 h after heat shock induction for in situ hybridization. DMSO-treated control embryos exhibited consistent expression of vimr1 during somitogenesis (Ua-d), whereas DMSO-treated HS:caβ-catenin embryos had decreased expression of the EMT marker in the tailbud (Va-d). Consistent with previous data (Fig. 3), FGF inhibitor-treated embryos had increased expression of vimr1 (Wa-d). HS:caβ-catenin embryos in the presence of SU5402 maintained strong expression of vimr1 (Xa-d). All tailbud images are lateral views, except where indicated otherwise. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Live imaging of tailbud EMT. (A-F) Transplanted HS:(CAAX)mCherry-p2a-(NLS)kikume cells were targeted to the NMP epithelium (A); the boxed region is shown in B. Time-lapse analysis shows that NMP epithelial cells undergo apical constriction and transition out of the epithelium but remain tethered to the epithelial layer by a stretched apical membrane (t=125 min; see also Movies 1 and 2; total of seven movies analyzed). The same embryo from A and B is shown in C and D at t=0 min (C) and t=350 min (D). Arrows (C,D) indicate cells that undergo the first EMT step over the course of the movie. Transplanted HS:TCFΔC cells heat shocked at the bud stage fail to leave the NMP epithelium (compare F with E; see Movie 3; total of four movies analyzed). (G-L) Cells that have left the NMP epithelium eventually migrate out of the tailbud into the PM territory (G,H, arrow), and produce extensive membrane protrusions during this process (I; see Movies 4 and 5). Cells lacking FGF signaling fail to directionally migrate out of the tailbud (J,K, arrow), but still exhibit extensive membrane protrusions (L; the cell in the center of panels is the same as that indicated by an arrow in J,K; see Movies 6 and 7; total of four movies analyzed). (M) Model for two-step EMT. In the zebrafish tailbud, Wnt initiates EMT in the sox2/ntla-positive NMP population through regulation of unknown target(s). This leads to movement into a transitional zone, where they express the EMT markers vimr1 and vimr2. Subsequently, FGF is required to terminate EMT and turn off early progenitor markers ntla/sox2 by promoting msgn1 and tbx16 expression. This results in exit from the tailbud and maturation of these cells to form the PM. |
|
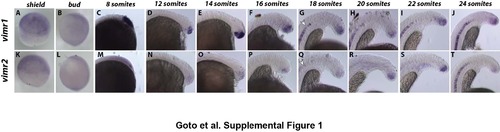
Time course expression of vimentin related genes Vimr1 (A-J) and Vimr2 (K-T) expression in wildtype embryos fixed at different stages. The beginning of notochord expression at the 18-somite stage is indicated by arrowheads (G and Q). Shield staged embryos were taken laterally with the shield towards the right. Embryos from 8- somite stage and onward were taken laterally with anterior towards the bottom. |
|
Expression of mesodermal maturation genes in FGF overactivated tailbud and expression domains of vimr1 and tbx16 in the wildtype tailbud. HS:cafgfr1 and wildtype embryos were heat-shocked at bud stage and fixed at 8-somite stage to examine the effect of FGF overactivation on maturation gene expression. Overactivation of FGF leads to strong expression of tbx16 and msgn1, which expands into the NMP region (C, C', D, D') compared to the control (A, A', B, B'). Double fluorescent in situ hybridization of tbx16 and vimr1 ina wild-type 20-somite stage embryo. As the mesodermal progenitors move from the dorsal region to ventral region, the cells turn on vimr1 and tbx16 (E, E', E'', E'''). However, as the cells enter the maturation zone in the lateral regions, the cells turn of vimr1 (F, F', F'', F'''). |