- Title
-
Infection of zebrafish embryos with live fluorescent Streptococcus pneumoniae as a real-time pneumococcal meningitis model
- Authors
- Jim, K.K., Engelen-Lee, J., van der Sar, A.M., Bitter, W., Brouwer, M.C., van der Ende, A., Veening, J.W., van de Beek, D., Vandenbroucke-Grauls, C.M.
- Source
- Full text @ J Neuroinflammation
|
Survival curves of 2 days post-fertilization embryos injected through different routes with wild-type Streptococcus pneumoniae D39. a Casper zebrafish embryo at 2 days post - fertilization. Red arrow indicates the hindbrain ventricle infection route, and black arrow indicates the caudal vein infection route. Scale bar, 500 µm. b, c Injection in the b hindbrain ventricle (HBV) or c caudal vein with indicated doses. Hpi hours post injection, CFU colony-forming units. The data represent the mean ± SEM of three individual experiments with 20 embryos in each group |
|
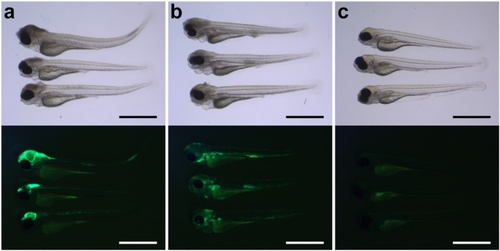
Bright-field images with corresponding fluorescent images of 2 day-post-fertilization zebrafish embryos infected by different routes. a, b Lateral view of zebrafish embryos injected in the a hindbrain ventricle or b in the caudal vein. c Non-injected control embryos. Please note that there is usually some background fluorescence observed in the yolk. All embryos were infected with 400 CFU of Streptococcus pneumoniae D39 (HlpA-GFP) and imaged at 48 h post injection. Scale bars, 500 µm |
|
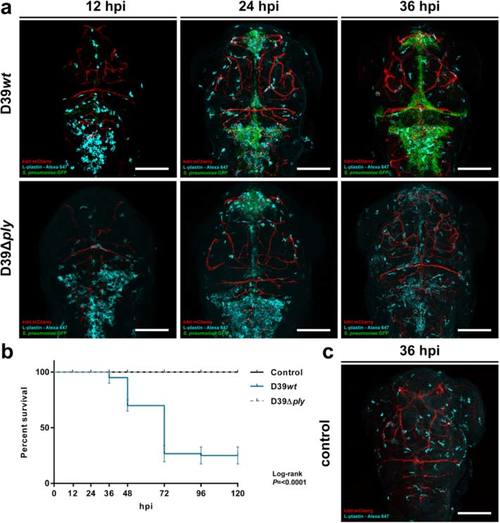
Comparison of 2 days post-fertilization zebrafish embryos infected with wild-type Streptococcus pneumoniae D39 (D39wt) or pneumolysin-deficient mutant strain (D39Δply). a Confocal microscopy images at maximum projection of Tg(kdrl:mcherry) s896 zebrafish embryos infected with D39wt or D39Δply at different time points. c Non-infected zebrafish embryos. D39wt pneumococci grow rapidly compared to D39Δply and migrate throughout the subarachnoid space, delineating the ventricular contours. The numbers of phagocytes reduce over time in the presence of increasing numbers of D39wt bacteria compared to non-infected or D39Δply zebrafish embryos. Embryos were infected with 600 CFU. Scale bars, 100 µm. b Corresponding survival curves. Embryos were infected with 300 CFU. The data represent three individual experiments with 20 embryos in each group |
|
Histopathological analysis of Streptococcus pneumoniae-infected zebrafish embryos via the hindbrain ventricle at 2 days post fertilization. a, b Sagittal section of the head region showing bacteria (arrow heads) in a the subarachnoid space and b brain parenchyma at 12 h post injection (hpi). c, d Sagittal section at 24 hpi showing increased amount of bacteria in the subarachnoid space (arrow heads) and disruption of the ventricular lining with bacterial infiltration (arrow) in c and a neutrophil (dotted circle) and a phagocytosing macrophage (circle) in d. Scale bars, 10 µm |
|
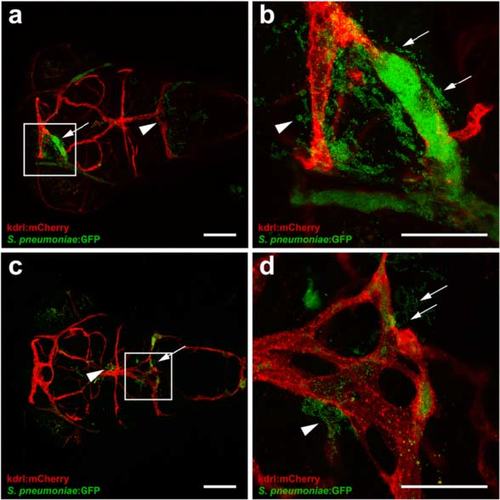
Pneumococci leave the blood vessels after systemic infection. a, b Confocal microscopy images at maximum projection of Tg(kdrl:mcherry) s896 zebrafish embryos injected in the caudal vein (CV) a before formation of the blood-brain barrier (BBB) at 2 days post fertilization (dpf) or b after the formation of the BBB at 4 dpf. Bacteria were localized inside (arrows) and outside (arrow heads) the blood vessels. All embryos were infected with 400 CFU and imaged at 24 h post injection. Scale bars, 50 µm |
|
Histopathological analysis of Streptococcus pneumoniae-infected zebrafish embryos via the caudal vein at 2 days post - fertilization. a, b Caudal vein injection at 2 days post - fertilization (dpf). Sagittal section of the head region showing the bacteria (arrows) in the brain parenchyma at a 12 h post injection (hpi) and at b 24 hpi. c, d Caudal vein-injected zebrafish embryos at 4 dpf. c Sagittal section at 24 hpi showing bacteria (arrows) in the meningeal space and in d the brain parenchyma. Scale bars, 10 µm |
|
Clogging of the blood vessels by Streptococcus pneumoniae after systemic infection. a-d Confocal microscopy images at maximum projection of Tg(kdrl:mcherry) s896 zebrafish embryos at 4 days post- fertilization injected in the caudal vein. a, c Bacteria were localized inside and outside of the blood vessels with (arrows) and without clogging (arrow heads). Scale bars, 100 µm. b, d An enlarged view of a and c, respectively, with clogging of a blood vessel highlighted. All embryos were infected with 600 CFU and imaged at 24 h post injection. Scale bars, 50 µm EXPRESSION / LABELING:
|
|
Time-lapse fluorescence imaging of Streptococcus pneumoniae-infected zebrafish embryos via the hindbrain ventricle at 2 days post fertilization. a-c Live fluorescence images of double-labelled Tg(mpx:GFP) i114 /Tg (mpeg1:mCherry) gl23 zebrafish embryos (green fluorescent neutrophils, red fluorescent macrophages). Dorsal view of the head region after injection with a PBS or b, c S. pneumoniae D39 with the corresponding Z-position (I) and bright-field image (II). Images b and c were acquired from the same zebrafish embryo at different positions. b (III) After injection of pneumococci in the hindbrain ventricle, green fluorescent neutrophils migrate in increasing numbers to the site of infection compared to a (III) PBS-injected zebrafish embryos. Macrophages were not observed in the subarachnoid space during b early pneumococcal infection of the hindbrain ventricle but c (III) remain localized in regions below the subarachnoid space. Scale bars, 100 µm |
|
Infection with green fluorescent wild-type HlpA-GFP Streptococcus pneumoniae D39 via the hindbrain ventricle can lead to systemic infection in zebrafish embryos. Fluorescence microscopy image at 36 h post injection. After infection of the embryos via the hindbrain ventricle, bacteria can disseminate into the bloodstream and cause a systemic infection. The embryo was infected at 2 days post-fertilization with 300 CFU of Streptococcus pneumoniae D39 (HlpA-GFP). Scale bar, 500 µm. |
|
Macrophages are involved in the clearance of pneumolysin-deficient pneumococci (D39Δply) in zebrafish embryos after infection via the hindbrain ventricle. Confocal microscopy images at maximum projection of double-labelled Tg(mpx:GFP) i114 /Tg (mpeg1:mCherry) gl23 zebrafish embryo (green fluorescent neutrophils (arrows), red fluorescent macrophages (arrowheads)) infected with 600 CFU Streptococcus pneumoniae D39Δply in the hindbrain ventricle at 2 days post fertilization and imaged at 24 h post injection. Scale bar, 50 µm. |