- Title
-
The endoderm indirectly influences morphogenetic movements of the zebrafish head kidney through the posterior cardinal vein and VegfC
- Authors
- Chou, C.W., Hsu, H.C., You, M.S., Lin, J., Liu, Y.W.
- Source
- Full text @ Sci. Rep.
|
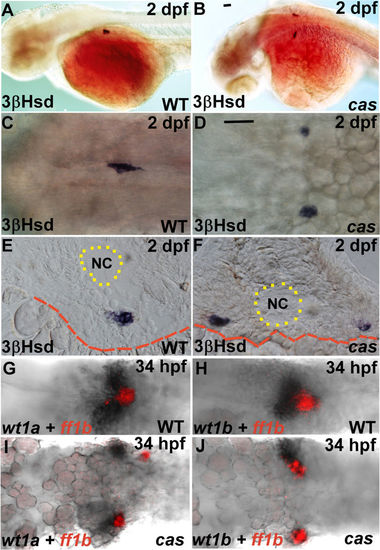
The phenotypes of the PG and the IR in the cas mutant and its wild-type sibling. (A–F) The steroidogenic IR detected by whole-mount 3β-Hsd staining at 2 dpf in the cas mutant (B,D,F) and its wild-type sibling (A,C,E). Dorsolateral (A,B) and dorsal (C,D) views are oriented with the anterior toward the left. (E,F) Cryosections with dorsal oriented toward the top. (G–J) Expression of ff1b and wt1a (G,I) or wt1b (H,J) were detected simultaneously in the cas mutant (I,J) and its wild-type sibling (G,H). The boundaries of the yolk and the notochord (NC) are highlighted by red dashed and dotted lines, respectively. Scale bar, 50 μm. |
|
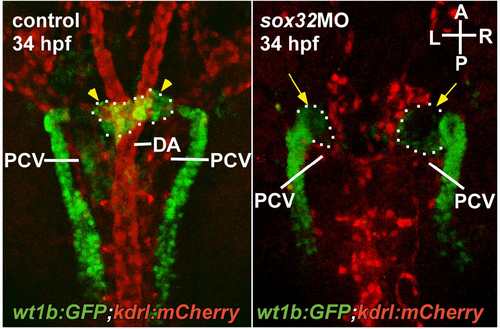
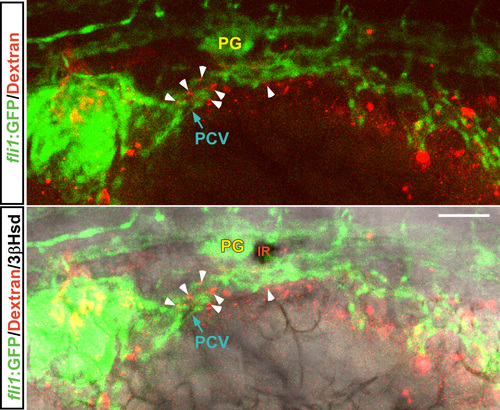
The morphology of the axial vasculature in the vicinity of the kidney and the IR in the sox32 morphant. 3β-Hsd activity staining was performed on the sox32MO-injected Tg(fli1:EGFP)y1 embryos (B–B'',D–D'',E–E'') and the uninjected (A–A'') or STD-MO-injected (C–C'') controls. All panels except (E–E'') are dorsal views with anterior oriented toward the top, while (E–E'') are dorsolateral views with anterior toward the left. The outlined area in E’ highlights the normal growth of the intersegmental vessels in the sox32 morphant. Yellow broken lines demarcate lateral boundaries of the PCV. Red arrows indicate IRs. Yellow arrowheads indicate PGs. Yellow arrows denote the pronephric tissues deficient in angiogenesis. Scale bar, 50 μm. |
|
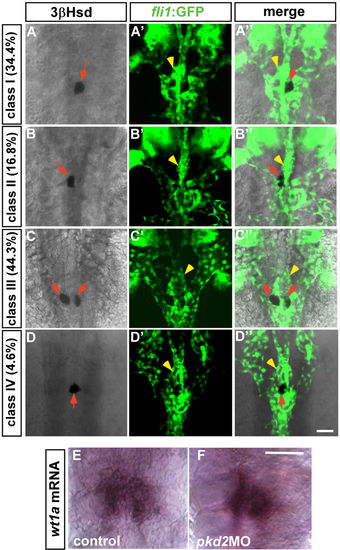
The phenotypes of the PG and the IR in the pkd2 morphant at 2 dpf. (A–D,A'–D',A''–D'') The Tg(fli1:EGFP)y1 embryos injected with the pkd2 MO displayed four classes of phenotypes at 2 dpf, which could be categorized according to various types of interrenal morphology. Class I: single IR cluster to the right of the midline (A–A''); Class II: single IR cluster to the left of the midline (B–B''); Class III: bilateral IR clusters immediately adjacent to the midline (C–C''); Class IV: single IR cluster at the midline (D–D''). There appeared to be no defects in glomerular angiogenesis (yellow arrowheads) in all classes. Red arrows indicate IRs. (E,F) Expression of wt1a was detected in the pkd2 morphant as well as the control embryo at 2 dpf. The images of the control embryo and the pkd2 morphant are representative of 23 and 13 samples, respectively. Scale bar, 50 μm. |
|
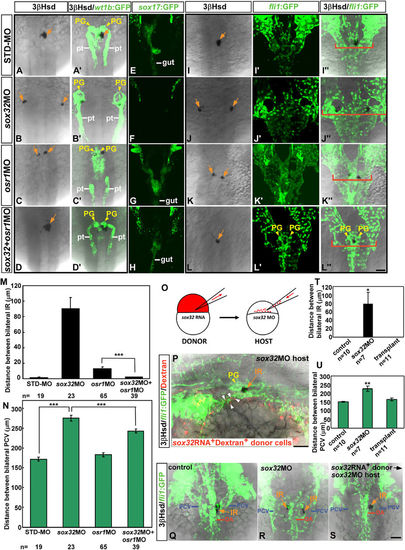
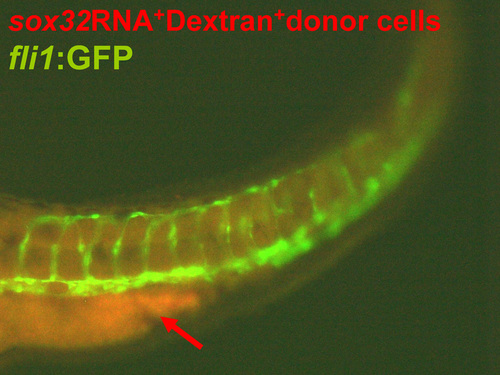
Reintroduction of endodermal cells into the endodermless embryo rescues defective migration of the head kidney and the PCV. (A–N) The inhibition of osr1 expression led to a rescue of renal, interrenal and PCV phenotypes in the sox32 morphant. Dorsal views of Tg(wt1b:GFP) (A–D,A'–D'), Tg(sox17:EGFP)s870(E–H) and Tg(fli1:EGFP)y1(I–L,I'–L',I”–L”) embryos injected with sox32MO, osr1MO, sox32/osr1 double-MOs or STD-MO, respectively, and harvested at either 34 hpf (A–D,A'–D',E–H) or 36 hpf (I–L,I'–L',I”–L”) for labelling steroidogenic IR (orange arrows) by 3β-Hsd activity staining. PG and pronephric tubules (pt) were detected by GFP expression in Tg(wt1b:GFP), while the gut tube was detected by GFP in Tg(sox17:EGFP)s870. The embryos are oriented with anterior to the top. Distances between bilateral IRs and the outer edges of the PCVs at the level of the IR as described in (I–L,I'–L') are quantified in (M,N). When the osr1 MO was co-injected with the sox32 MO, the convergence defects of bilateral IRs and PCVs were significantly alleviated. Red brackets mark lateral boundaries of the PCV branches at the level of the IR. (O–U) Transplantation of sox32-expressing cells into the sox32 morphant rescued the defective migration of bilateral PCVs and IRs. (O) Schematic of the transplantation approach using Sox32 to target donor cells to the endoderm. (P) The lateral view of a representative recipient (n = 11/12) shows that grafted sox32RNA+Dextran+ cells were associated with the PCV endothelium. The sox32RNA+Dextran+ cells near the PCV are marked by white arrowheads. The embryo is oriented with anterior to the left. Dorsal views of the IR and the PCV in the control, the sox32 morphant and the recipient are shown in (Q–S), and the distance between bilateral IRs and PCVs are quantified in (T,U) respectively. *P < 0.05; **P < 0.005; ***P < 0.001 (Student's t-test). Scale bar, 50 μm. |
|
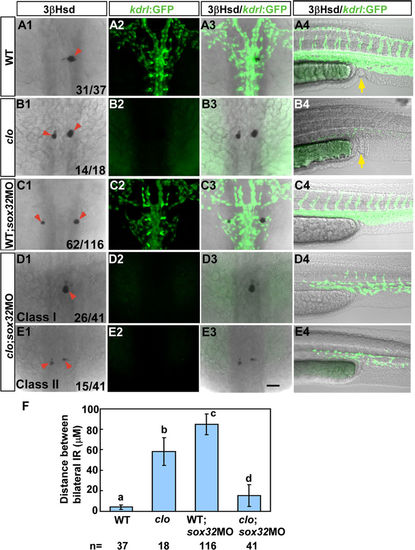
The defective interrenal migration in the sox32 morphant is suppressed by the clom39 mutation. The sox32MO was injected into the Tg(kdrl:EGFP)s843; clom39 mutant (D1–D4,E1–E4) and its wild-type sibling (C1–C4) to inhibit endoderm formation, as verified by the absence of anal openings (C4–E4). By contrast, the clom39 mutant (B1–B4) and its wild-type sibling (A1–A4) showed normal formation of anal openings (yellow arrows in A4 and B4). All images are single confocal sections showing the IR as detected by 3β-Hsd staining (A1–E1,A3–E3) and the vascular pattern at the midtrunk (A2–E2,A3–E3) and the lower trunk (A4–E4) revealed by GFP in Tg(kdrl:EGFP)s843 at 34 hpf. In clom39, the endothelial fluorescence was absent at the midtrunk and disrupted at the lower trunk. The severe disruption of interrenal migration in the sox32 morphant was rescued when the endothelium was simultaneously deleted by the clom39 mutation, as revealed in both class I (D1–D3) and class II (E1–E3) double loss-of-function embryos. The effects of various treatments on the convergence of bilateral IRs are quantified in (F). Histograms with different letters above them are significantly different (ANOVA and Duncan’s multiple test, P < 0.05). Scale bar, 50 μm. |
|
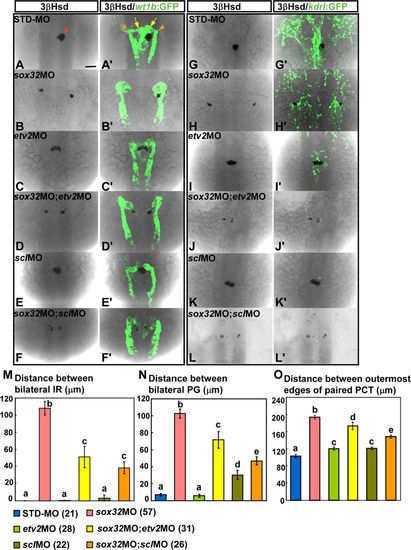
The defective midline convergence of the PG and the IR in the sox32 morphant at 34 hpf is suppressed by both etv2MO and sclMO. sox32MO, etv2MO, sclMO, sox32/etv2 double-MOs, sox32/scl double-MOs and STD-MO were injected into either Tg(wt1b:GFP) (A–F,A'–F') or Tg(kdrl:EGFP)s843 (G–L,G'–L') embryos. The injected embryos were subjected to 3β-Hsd staining and analysed for the effects of MOs on the morphology of the kidney delineated by wt1b:GFP (A'–F'), the axial vasculature by kdrl:EGFP (G'–L'), and the IR by 3β-Hsd (A–L). Distances between bilateral IRs, bilateral glomeruli and the outermost edges of paired PCTs in injected Tg(wt1b:GFP) embryos are quantified in (M–O), respectively, with the number of samples indicated in parentheses. The IR, the glomeruli and the PCT are denoted by red arrow, yellow arrows and orange arrowheads, respectively. Histograms with different letters above them are significantly different (ANOVA and Duncan's multiple test, P < 0.05). Scale bar, 50 μm. |
|
Both vegfCMO and flt4MO rescue the convergence of the IR in sox32-deficient embryos. (A) (Upper) Colocalization of vegfC with wt1b and ff1b, respectively, in the 25 hpf embryo by ISH. (Lower) A cross-section of a 34 hpf embryo showing that VegfC protein (red) was enriched in the kidney (delineated by wt1b:GFP). (B) sox32MO, vegfCMO, flt4MO, sox32/vegfC double-MOs, sox32/flt4 double-MOs were injected into Tg(kdrl:EGFP)s843 embryos. The injected embryos were subjected to 3β-Hsd staining and analysed for the effects of MOs on the morphology of the IR (delineated by the 3β-Hsd activity and marked by orange arrows) and the axial vasculature (delineated by kdrl:EGFP). Dorsal views, with anterior to the top. Distances between bilateral IRs and between the bilateral edges of the PCV at the level of the IR (marked by red brackets) are quantified in (C). (D) Dorsolateral views of the control, vegfCMO-injected, and flt4MO-injected Tg(kdrl:EGFP)s843 embryos at 34 hpf, with anterior to the left, to show the relative distribution of the DA, PCV and IR. Scale bar, 50 μm. |
|
The relative spatial distribution of the PG, the IR, the DA, the PCV, the pronephric tubules (PT), and the CCV is shown by (A) a dorsolateral view of the 3β-Hsd activity stained Tg(wt1b:GFP); Tg(kdrl:mCherry)ci5 embryo oriented with anterior to the right and (B) a schematic depicting the dorsal view of head kidney structures and axial vessels at the stage of 34 hpf and 2 dpf respectively. D, dorsal; V, ventral; A, anterior; P, posterior; R, right; L, left. |
|
Angiogenesis of the PG was defective in the sox32 morphant. The pronephric kidney and the blood vessels are delineated by green and red fluorescence respectively in the Tg(wt1b:GFP);Tg(kdrl:mCherry)ci5 embryo at the stage of 34 hpf. The relative spatial distribution of the PG (yellow arrowheads), the DA and the PCV is shown in a dorsal view. Yellow arrows indicate defective angiogenesis of the PG. A, anterior; P, posterior; R, right; L, left. |
|
The curly up tail phenotype of the Tg(fli1:EGFP)y1 embryo injected with the pkd2 morpholino. Scale bar, 50 μm. |
|
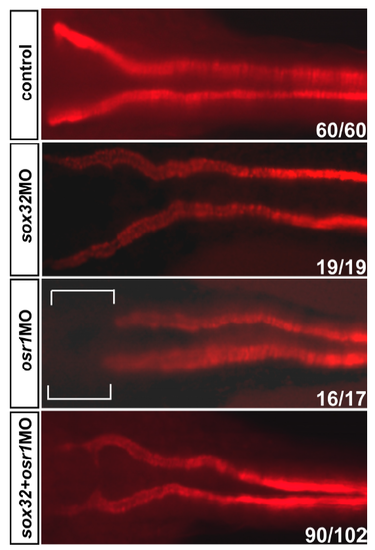
Compromised formation of proximal convoluted tubules at 53 hpf in the osr1 morphant was rescued upon a co-injection of sox32MO. The pronephric tubules were labelled by the α6F antibody that detects Na+/K+ATPase. The bracketed region in the osr1 morphant denotes a loss of proximal convoluted tubules. |
|
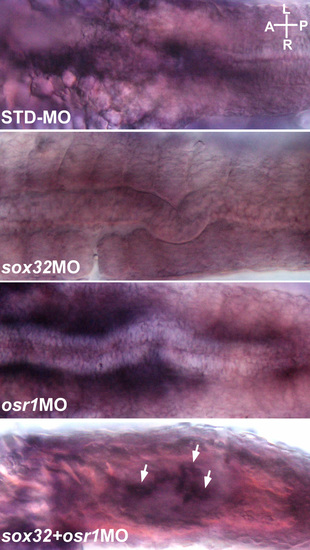
Ventral views of the embryos injected with STDMO, sox32MO, osr1MO and sox32/osr1MO, respectively, and analysed by ISH for detecting the foxa2 expression at the midtrunk at 24 hpf. White arrows indicate the rescued foxa2 expression in the sox32/osr1 double morphant. The images shown are representative of 19, 15, 17 and 23 samples respectively. A, anterior; P, posterior; R, right; L, left. |
|
A magnified dorsolateral view of the transplanted embryo shown in Figure 4P. Grafted sox32RNA+Dextran+ cells (marked by white arrowheads) were associated with the PCV endothelium in the |
|
The donor cells from the embryo injected with sox32 mRNA and fluorescent dextran developed into a gut tube-like structure at the posterior trunk of the sox32MO-injected host embryo. |
|
The endoderm formation delineated by the fluorescence of Tg(sox17:EGFP)s870 was inhibited by the sox32MO, which was injected individually or co-injected with either etv2MO or sclMO. |
|
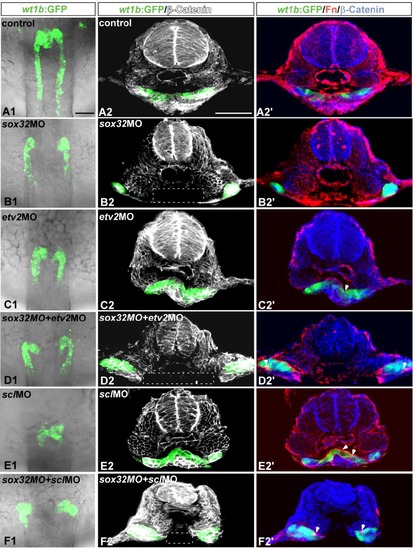
The defective midline convergence of the kidney in the sox32 morphant prior to interrenal differentiation was not suppressed by either etv2MO or sclMO. sox32MO, etv2MO, sclMO, sox32/etv2 double-MOs, sox32/scl double- MOs and STD-MO were injected into Tg(wt1b:GFP) embryos to test their effects on kidney morphology at 24 hpf. (A1-F1) The kidney morphology was delineated by the expression of wt1b:GFP in the dorsal whole-mount views of the midtrunk. (A2-F2) Cross-sections showing the expression of wt1b:GFP and β-Catenin (white). (A2'-F2') Cross-sections showing the expression of wt1b:GFP, Fn (red) and β-Catenin (blue). The loss of β-Catenin in the ventral region is highlighted by white rectangles, and Fn deposits are marked by white arrows. The images shown are representative of 3, 15, 20, 16, 20 and 11 samples for control embryos, sox32 morphants, etv2 morphants, etv2/sox32 double morphants, scl morphants and scl/sox32 double morphants, respectively. Scale bar, 50 μm. |
|
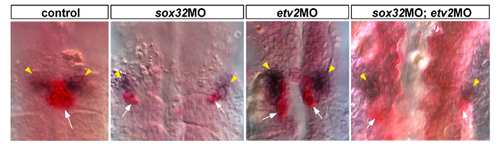
Expression patterns of ff1b (red) and wt1b (dark purple) were detected by double ISH in the sox32 morphant, the etv2 morphant, the sox32/etv2 double morphant and the control embryo at 24 hpf. Localizations of the PG (marked by yellow arrowheads) and the IR (marked by white arrows) are viewed ventrally. |