- Title
-
Myeloid Growth Factors Promote Resistance to Mycobacterial Infection by Curtailing Granuloma Necrosis through Macrophage Replenishment
- Authors
- Pagán, A.J., Yang, C.T., Cameron, J., Swaim, L.E., Ellett, F., Lieschke, G.J., Ramakrishnan, L.
- Source
- Full text @ Cell Host Microbe
|
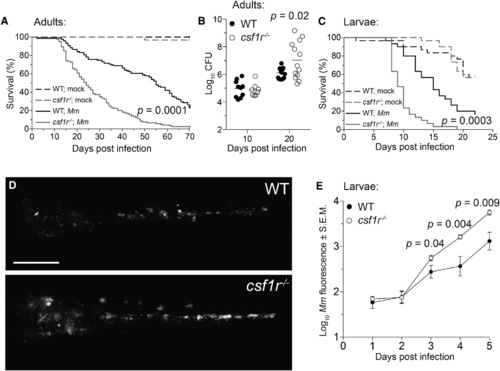
csf1r Mutant Zebrafish Are Hypersusceptible to M. marinum Infection (A) Survival of adult zebrafish injected intraperitoneally with ~273 M. marinum or an equivalent volume of PBS (mock). n = 30 for each group. (B) Bacterial burdens (CFU, colony-forming units) of adult zebrafish infected intraperitoneally with ~115 CFU of M. marinum. Horizontal lines indicate mean values. (C) Survival of zebrafish larvae injected with PBS (mock) or ~193 M. marinum via the caudal vein. n = 30 for each group. (D and E) Representative images (D) and mean bacterial burden (E) of larvae infected with ~200 fluorescent M. marinum via the caudal vein. Scale bar, 300 µm. Error bars indicate standard error of the mean (SEM). Statistical significance was determined by log-rank test (A, C) or two-tailed unpaired Student’s t test (B, E). Data are representative of more than three experiments. See also Figures S1-S3 and Movies S1 and S2. |
|
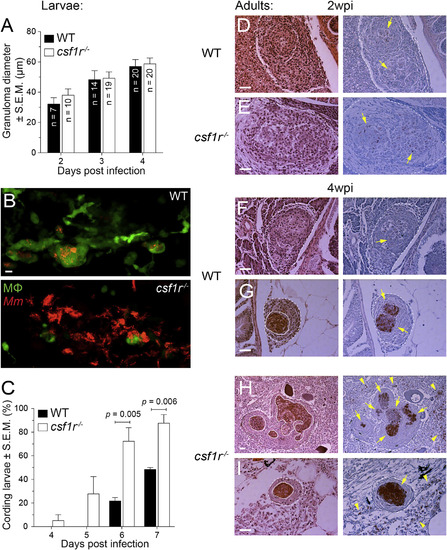
Exuberant Mycobacterial Growth in csf1r Mutants Is Associated with Granuloma Necrosis (A) Mean granuloma diameter measured by their longest axis in larvae infected at 2 dpf via the caudal vein with ~195 tdTomato-expressing M. marinum. Data are representative of two sets of injections. (B) Maximum-intensity projections of macrophage-replete WT granuloma or a macrophage-depleted csf1r mutant granuloma showing early signs of cording in 5 dpi mpeg1:YFP larvae infected at 2 dpf via the caudal vein with ~200 tdTomato-expressing M. marinum. Scale bar, 10 µm. (C) Percentage of larvae with cording phenotype after infection with ~190 M. marinum via the caudal vein. Average values from four separate sets of injections from at least two independent experiments were plotted. In (A) and (C), error bars indicate SEM. (D-I) Representative hematoxylin and eosin (left) and modified Ziehl-Neelsen (right) stains of wild-type or csf1r mutant adult zebrafish 2 weeks (D and E) or 4 weeks (F–I) post-intraperitoneal infection with ~100 CFU of M. marinum. Dotted lines delineate granulomas and solid lines indicate regions of necrosis. Arrows indicate mycobacteria inside granulomas and arrowheads indicate mycobacteria outside of granulomas. Scale bars, 50 µm. Two or three fish per group were used for each time point. EXPRESSION / LABELING:
|
|
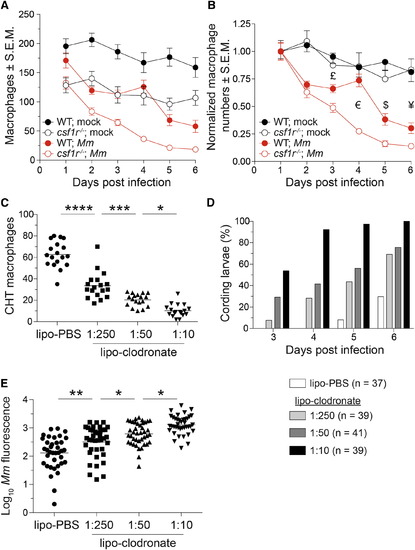
A Baseline Macrophage Deficit Is Associated with an Accelerated Depletion of the Granuloma Macrophage Supply (A and B) Absolute numbers (A) or normalized numbers (B) of macrophages in mpeg1:Gal4FF; UAS:E1bKaede larvae infected with ~240 M. marinum or mock injected. n = 5-9 larvae per group. Error bars indicate SEM. (C) Number of macrophages in the CHT of 4-dpf mpeg1:YFP larvae 40 hr after injection of 1:10 dilution of lipo-PBS or graded doses of lipo-clodronate. (D and E) Bacterial kinetics of cording (D) and burdens (E) in larvae infected with tdTomato-expressing M. marinum via the caudal vein at 2 dpf. Statistical significance was determined by two-tailed unpaired Student’s t test (B) or one-way ANOVA with Sidak’s post-test (C and E). £, p = 0.02; €, p = 3 × 10-8; $, p = 0.004; and ¥, p = 0.03. (C-E) Data are representative of three independent experiments. See also Figure S4. |
|
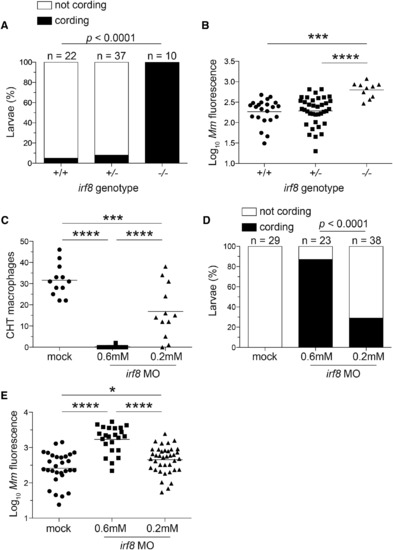
Macrophage Depletion Caused by irf8 Deficiency Promotes Granuloma Necrosis (A-E) Larvae from an irf8st96/+ intercross were infected via the caudal vein with ~200 tdTomato-expressing M. marinum. (A) Percentage of larvae with cording phenotype and (B) bacterial loads 3 dpi. (C) Macrophage numbers in irf8 morpholino (MO)- and mock-injected mpeg1:YFP larvae 2 dpf. (D) Bacterial cording and (E) bacterial burdens in irf8 and mock morphants 3 dpi with ~300 tdTomato-expressing M. marinum injected via the caudal vein. (B, C, E, and G) Each symbol represents individual larvae, and horizontal lines indicate means. Statistical significance was determined by Fisher’s exact test (A and D) or one-way ANOVA with Tukey’s post-test (B, C, and E). Data are representative of three (A-D) experiments. PHENOTYPE:
|
|
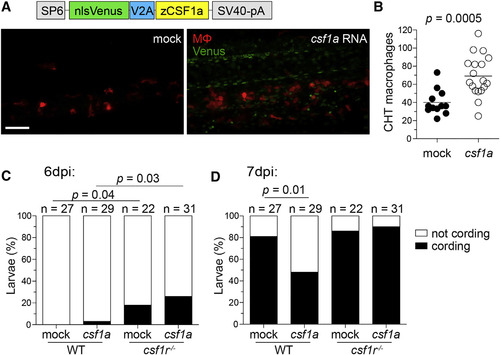
Increasing Macrophage Supply in Wild-Type Zebrafish Delays Granuloma Necrosis (A-D) One-cell stage WT mpeg1:tdTomato embryos were injected with vehicle (mock) or ~2 nl of 200 ng/µl in vitro-transcribed csf1a RNA. (A) Schematic of csf1a overexpression construct and maximum intensity projections of the caudal hematopoietic tissue of 2-day-old larvae. Scale bar, 100 µm. (B) Number of macrophages in the CHT of 2 dpf larvae. Horizontal lines depict means. Percentage of cording in WT or csf1r mutant larvae 6 dpi (C) or 7 dpi (D) with ~200 M. marinum expressing tdKatushka2. Statistical significance determined by two-tailed unpaired Student’s t test (B) or Fisher’s exact test (C and D). EXPRESSION / LABELING:
|
|
Restoring CSF-1R Signaling during an Ongoing Infection Delays Granuloma Necrosis (A) Schematic of temperature-shifting experiment. (B-D) Macrophage numbers (B), bacterial cording (C), and bacterial burdens (D) in phenotypically WT heterozygotes (csf1rts/+), csf1r null homozygous mutants (csf1r-/-), and temperature-sensitive heterozygotes (csf1rts/-). Horizontal lines indicate means. Statistical significance was determined by one-way ANOVA with Tukey’s post-test (B and D) or Fisher’s exact test, correcting for multiple comparisons by multiplying p values obtained in pairwise comparisons by the number of groups in each experimental condition (C). |
|
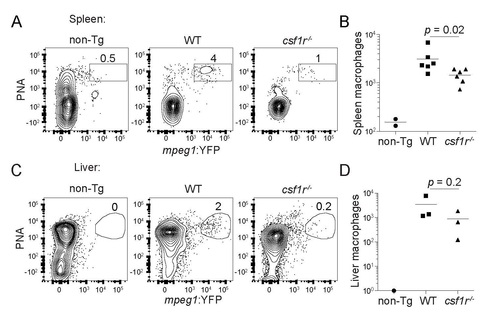
(Related to Figure 1). Adult csf1r mutant zebrafish have impaired macrophage development. (A) Flow cytometry plots and (B) absolute numbers of viable (DAPI-) splenic macrophages in 3-4 month-old wild-type (WT) and csf1r-/- mpeg1:YFP fish. mpeg1:YFP- WT (non-transgenic, non-Tg) fish were used as negative controls. Each symbol represents individual larvae. (C) Flow cytometry plots and (D) absolute numbers of viable (DAPI-) liver macrophages in 3-4 month-old wild-type (WT) and csf1r-/- mpeg1:YFP. Numbers above gated populations indicate percentages within parent population. Data are representative of two independent experiments (A) or were pooled from two experiments (B). Statistical significance determined by two-tailed unpaired Student’s t test. PHENOTYPE:
|
|
(Related to Figure 1). Larval csf1r mutant zebrafish have impaired macrophage development. Enumeration of larval macrophages in wild-type (WT) and csf1r-/- mpeg1:YFP fish. (A) Maximum intensity projections of the hindbrains of WT and csf1r-/- mpeg1:YFP larvae at 3dpf. Scale bars, 100µm. Number of macrophages in the (B) midbrain and (C) CHT of 3dpf fish. (D) Absolute numbers of macrophages at 6dpf. Number of neutrophils in the CHT of 3dpf fish as determined by Sudan Black staining. (B - E) Each symbol represents individual larvae. (B - D) Data are representative of at least two experiments. (F - H) Assessment of macrophage motility: (F) Representative maximum intensity projections of the CHT of WT and csf1r-/- mpeg1:YFP larvae at 6dpf and (G) average sphericity and (H) speed of individual macrophage tracks. Hashed boxed indicate zoomed region displayed on right corner. Scale bar, 40µm. Horizontal lines indicate means (B - E) or median values (G, H). Statistical significance determined by two-tailed unpaired Student’s t test (B - E) or Mann-Whitney U test (G, H). EXPRESSION / LABELING:
PHENOTYPE:
|