- Title
-
Functional analyses of variants reveal a significant role for dominant negative and common alleles in oligogenic Bardet-Biedl syndrome
- Authors
- Zaghloul, N.A., Liu, Y., Gerdes, J.M., Gascue, C., Oh, E.C., Leitch, C.C., Bromberg, Y., Binkley, J., Leibel, R.L., Sidow, A., Badano, J.L., and Katsanis, N.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
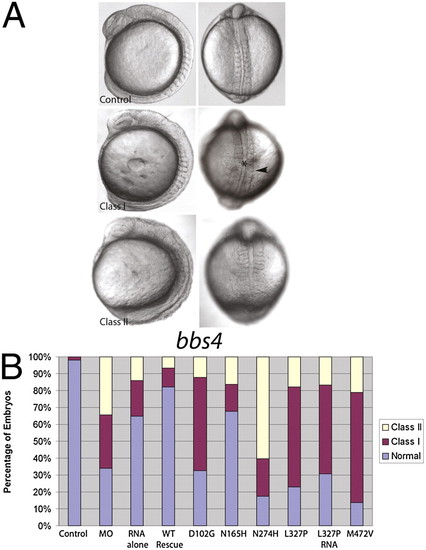
Suppression of BBS4 produces specific defects. (A) Injection of MOs against individual bbs genes (bbs4 shown here) into zebrafish embryos produces gastrulation defects, including a short body axis, a widened and kinked notochord (*), and broadened somites (arrowhead), which can be rescued by WT human mRNA. Coinjections of mutant mRNAs produce a spectrum of defects. (B) Whereas WT RNA rescues the MO phenotypes (WT RNA), coinjection of hypomorphic mutations (N165H) partially rescues the phenotype; however, null mutations (D102G, N274H, and M472V in BBS4) do not rescue, and dominant-negative mutations (L327P) exacerbate the phenotype and produce defects by injection of RNA alone. PHENOTYPE:
|
|
In vitro validation of mutation effects. (A–C) Localization of myc-tagged WT BBS4 protein (red) in IMCD3 cells shows presence at the basal body, as indicated by colocalization with γ-tubulin and acetylated α-tubulin (green). (D–F) Introduction of an artificial N165F mutation in BBS4 has no observable effect on localization. Expression of the D102G mutant, characterized as null by in vivo scoring, is undetectable 2 d after transfection (G–I), whereas the hypomorphic N165H variant is expressed but mislocalized (J–L). The dominant-negative mutation L327P results in mislocalization in postmitotic cells (M–O) but not in dividing cells (P–R). (S) Expression of MYC-tagged constructs 4 d after transfection is undetectable for null mutations (D102G, N274H, and N165H), although RT-PCR showed message in each case. |
|
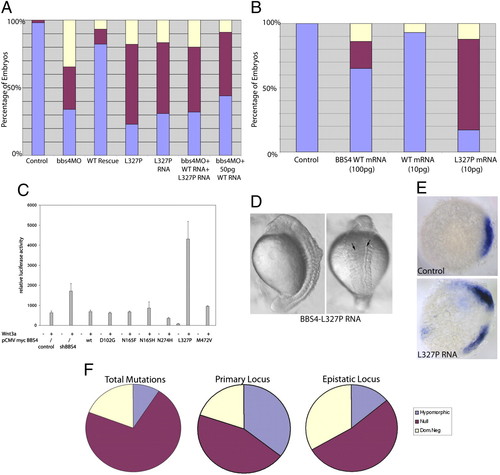
The dominant-negative effect of BBS4 L327P. (A) Coinjection of 3 ng of bbs4 MO and 100 pg of WT human mRNA rescues the morphant phenotype, and coinjection of 100 pg of L237P bearing BBS4 mRNA produces defects similar to, or more severe than, MO alone. Introduction of equimolar amounts (50 pg each), however, of both WT and mutant mRNA attenuates the ability of 50 pg of WT mRNA alone to rescue partially the morphant phenotype. (B) Injection of a suboptimal dose (10 pg) of L327P mRNA produces significant phenotypes. (C) Overexpression of the dominant-negative BBS4 mutation L327P but not of the loss-of-function alleles activated canonical Wnt signaling by the Super TOP-Flash luciferase assay in a manner similar to BBS4 suppression. Overexpression of L327P mRNA at a high concentration (300 pg) results in dorsalized embryos and double axes (arrows) (D) and ectopic chordin expression (E). (F) In the context of oligogenic inheritance, the primary (recessive) locus has a significantly increased likelihood of being hypomorphic (P < 0.0001), whereas the epistatic locus is more likely to be severe, with significant enrichment of dominant-negative lesions (P = 0.0004). |
|
Reevaluation of genetic data using in vivo scoring. Effects of mutations based on in vivo analyses suggest that the severity of BBS mutations is consistent with segregation of disease in families with BBS. (A) Although the heterozygous nonsense BBS1 mutation Q128X present in the individual who has BBS in family AR186 is functionally null (B and C), its presence does not fully account for the disease. (B and C) Affected individual, however, is also homozygous for the BBS2 polymorphism, I123V, predicted to be a mild hypomorph. (D) Injection of either bbs1 or bbs2 MO alone at suboptimal concentrations results in mildly affected embryos. Coinjection of both MOs together produces severely affected embryos with phenotypes not seen by injection of either MO alone, including near-complete loss of somite definition (labeled by myoD) and loss of eye field (labeled by pax2). PHENOTYPE:
|
|
Suppression of bbs genes in vivo produces early gastrulation phenotypes. (A) Injection of splice-blocking (SB) MO against bbs1 or bbs6 results in complete suppression of message as shown by PCR amplification of cDNA reverse transcribed from extracted total mRNA. β-actin was used as a control. (B) SB MO produces similar phenotypes to those produced by translation-blocking MO and in similar proportions (C). Consistent with these similar defects, co-injection of SB MO with mutant mRNA for a subset of bbs1 or bbs6 mutations produced defects similar to those produced by coinjection with TB MO. (D-F) To verify specificity of each BBS mRNA to rescue the corresponding bbs MO, we attempted to rescue bbs1, bbs7, bbs4, bbs6, or bbs3 with either bbs7, bbs1, bbs6, or bbs4, respectively, and found that the mismatched mRNA did not rescue the morphant phenotypes. PHENOTYPE:
|
|
Quantification of somite stage phenotypes. Embryos co-injected with bbs MO and WT or mutant or mRNA were stained by in situ hybridization with pax2/krox20/myoD. Trunk length was measured from the first to the last appreciable somite to determine the extent of the shortened body axis phenotype. Embryos shown are representative examples of benign, hypomorph, null and dominant Negative mutations. (A) MO injected embryos were significantly shorter than uninjected control embryos, but co-injection of WT mRNA rescued the length defect. Consistently, mutations predicted null or dominant negative by live embryo scoring resulted in body length similar to morphants (e.g. BBS10 Y197C or BBS2 N70S, respectively) while those predicted to be Benign were similar to uninjected controls (e.g. BBS6 R518H). (B) The normal distribution of trunk length measurements shows the clustering of groups of effects for BBS1 and 2 (B), BBS3 and 6 (C) and BBS10 and 12 (D). Uninjected controls and WT rescue embryos are consistently longer, while MO and null and dominant negative effect mutations are consistently shorter (p≤0.001). Hypomorphic mutations (e.g. BBS12 A289P) are between the two. |
|
Quantification of gastrulation defects. (A) Movement of cells during gastrulation stages is defective in MO-injected and null or dominant negative mutant rescued embryos, whereas WT rescued embryos are similar to uninjected controls. (B-G) The extent of gastrulation movements was quantified by measurement of the width of the field of fluorescing cells, repeated in triplicate – error bars shown. Dominant negative mutations, such as BBS2 N70S (C) and BBS3 L170W (D), produce cell movement defects more severe than MO alone, while null mutations such as BBS10 Y197C (F) are similar to MO. Hypomorphic mutations, such as BBS1 M390R (B) and BBS12 A289P (G) are less severe than MO, but do not efficiently rescue the gastrulation movement phenotype. PHENOTYPE:
|
|
Rescue of bbs morphants with human BBS mRNA. Translation blocking morpholinos for bbs1-12 were injected into zebrafish embryos and produced early embryonic defects including shortened body axis and abnormal somite and notochord morphology. These defects could be rescued with co-injection of human WT mRNA encoding the respective BBS gene. Co-injection with mRNA baring mutations producing a spectrum of effects ranging from rescue similar to WT rescue, such as BBS6 R518H (R), or partial rescue, as with the Hypomorph BBS1 M390R (C), or lack of rescue, such as the Null BBS10 Y197C (D′), or exacerbation of the phenotype, such as the Dominant negative BBS3 G169A (I). PHENOTYPE:
|
|
In vivo assessment of missense polymorphisms reveals pathogenic effects. 17 polymorphisms were tested by in vivo mRNA rescue of MO injection. While several (n=7) rescued the morphant phenotype as efficiently as WT mRNA, suggestive of Benign variants (e.g. BBS12 R386Q and S616N in F), others produced pathogenic defects ranging from Hypomorphic (BBS2 I123V in C) to Null (BBS10 N544S in E) to dominant negative (BBS2 V122I in C). |
|
In vitro assessment of mutation effects. Localization of MYC-tagged WT BBS proteins (red) and GFP (green) in IMCD3 cells reveal that most WT proteins localize to the cytoplasm (BBS1, BBS2, BBS3, BBS6, and BBS7). Null mutations show reduced expression (BBS6 T57A; I), while dominant negative mutations seem to drive protein localization to cellular structures not seen in WT expression, such as BBS2 N70S (D) or BBS7 T211I and BBS7 H323R expression in the nucleus (M-N). (O) Expression of RNA from each transfected construct was verified by RT-PCR analysis. |
|
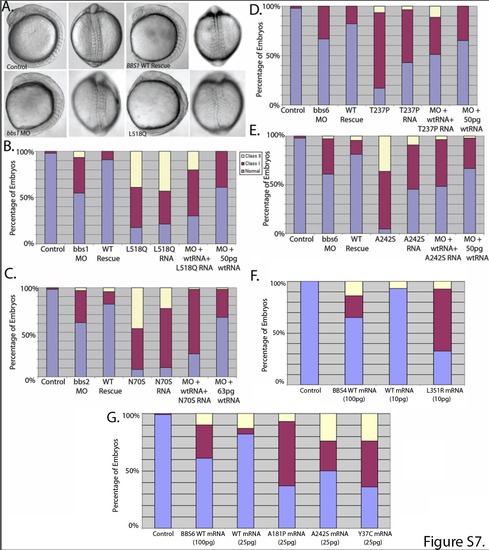
Verification of dominant-negative mutations. (A-B) While co-injection of 3ng of bbs1 MO and 100pg of WT human mRNA rescues the morphant phenotype, coinjection of 100pg of BBS1 mRNA encoding the dominant negative mutation, L518Q produces defects similar to, or more severe than, MO-alone. (B) Further, injection of an equimolar amount of mutant and WT mRNA (50pg each) attenuates the ability of 50pg of the WT mRNA alone to partially rescue the morphant phenotype. (C-E) This was also the case for other dominant negative mutations tested in BBS2 and BBS6. Further investigation of dominant negative alleles revealed that, when mutant mRNA is injected at a suboptimal concentration (i.e. one producing minimal defects when used for WT mRNA), BBS4 (F) and BBS6 (G) alleles produce significantly deleterious effects. |