- Title
-
The Zebrafish Orthologue of the Dyslexia Candidate Gene DYX1C1 Is Essential for Cilia Growth and Function
- Authors
- Chandrasekar, G., Vesterlund, L., Hultenby, K., Tapia-Páez, I., and Kere, J.
- Source
- Full text @ PLoS One
|
Expression of dyx1c1 mRNA during embryonic development and in adult tissues. qPCR analysis of the transcript levels of dyx1c1 during embryonic development (A) and in adult tissues (B). Whole-mount in situ hybridization showed that dyx1c1 is expressed in KV at 10 hpf (C). Inset in C is a close-up view of KV. At 15-somites, dyx1c1 was expressed specifically in the otic vesicle, pronephros and neural tube (D). At 26 hpf dyx1c1 was detected in the brain and is still maintained in otic vesicle, pronephros and spinal canal (E–G). Later at 49 hpf, dyx1c1 was visible in the olfactory placode (H). Panels E–G show lateral views of embryos. Panels D and H show dorsal and ventral views of embryos, respectively. Scale bars indicate 100 μm. Abbreviations: KV, Kupffer’s vesicle: nt, neural tube: pn, pronephros: t, telencephalon: d, diencephalon: m, midbrain: tg, tegmentum: ov, otic vesicle: sc, spinal canal: op, olfactory placode. |
|
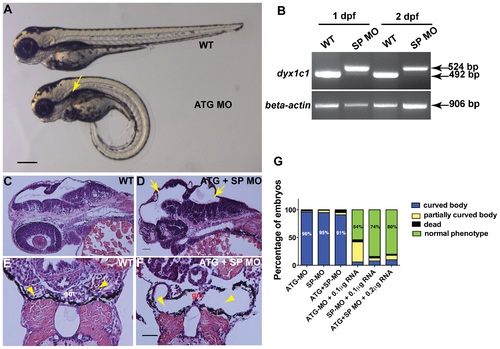
Knockdown of dyx1c1 showed typical cilia phenotypes. Injection of ATGMO or SPMO at 200 µM concentration produced ventrally curved body axis, hydrocephalus and kidney cysts (A). Arrow in panel A denotes kidney cyst in ATG morphant. RT-PCR showed aberrant splice transcripts in SPMO injected embryos at 1 and 2 dpf (B). Histological sections of 2 day old embryos injected with both ATGMO and SPMO (100 µM each) showed hydrocephalus (D; yellow arrows) compared to normal size brain ventricles in WT (C). Transverse histological sections across the pronephros at 3.5 dpf showed normal pronephros in WT embryos (E). Section of dyx1c1 morphant (ATGMO+SPMO) showed severe pronephric distention and a thin glomerulus in the center (F). Yellow arrowheads in panel E and F point out normal pronephros in WT and dilated pronephros in morphant embryo, respectively. Quantitative analysis of the rescue of dyx1c1 morphant phenotype to WT phenotype with different combinations of MOs and dyx1c1 mRNA (G). Scale bars indicate 100 µm. Abbreviation: gm, glomerulus. PHENOTYPE:
|
|
Loss-of-function of dyx1c1 caused L–R assymetry defects in zebrafish. Whole-mount in situ hybridization showing the expression of laterality markers in WT and dyx1c1 morphants (ATGMO+SPMO) at 2 dpf. The position of the asymmetrically placed organs was irregular in at least 50% of the embryos injected with dyx1c1 MO. otx5 expression showing the left sided placement of the parapineal organ in WT (A). In dyx1c1 morphant, the parapineal position was reversed (B). lov was strongly expressed in the left habenular in WT (C). In dyx1c1 morphant lov expression was stronger in the right or expressed symmetrically (D & E). Knockdown of dyx1c1 altered heart looping. Ventral views of embryos at 2 dpf (F–H). cmlc2 expression showed normal heart looping in WT (F). Cardiac looping was reversed (G) or absent in dyx1c1 morphant embryos (H). Expression of foxa3 revealing the position of the gut, liver and pancreas in WT embryo (I). The positions of the liver, gut and pancreas were irregular in dyx1c1 morphants (J & K). Panels A, B, C, D, E, I, J and K are dorsal views with anterior to the top. Frontal views are shown in panels F–G. Scale bars indicate 50 μm. Abbreviations: p, pineal organ: pp, parapineal organ: v, ventricle: a, atrium: li, liver: pa, pancreas: g, gut. |
|
Knockdown of dyx1c1 reduced cilia length and number in different organs Immunolabelling of KV cilia with anti-acetylated tubulin in WT (A) and dyx1c1 morphants (B) at 14 hpf showed reduction in cilia length in morphant embryos. Compared to WT control (C), morphant embryo (D) revealed fewer and shortened cilia in the olfactory placode. At 2 dpf, cilia in dyx1c1 morphants were shortened in pronephros and spinal canal (F, H) compared to WT (E, G). Measurement of cilia number in WT and morphants (I). Confocal images of KV in WT and morphant embryos at 14 hpf stained with anti-acetylated tubulin and DAPI (J, K). Graphical representation of total number of nuclei/KV in WT and morphant embryos (L). Panel C, D, E, F, G and H are lateral views with anterior to the left in E, F, G and H. Scale bars indicate 10 μm. PHENOTYPE:
|
|
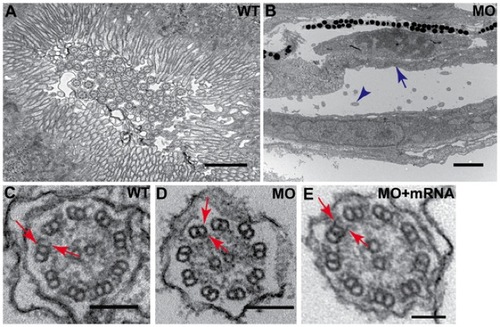
Disruption of dyx1c1 leads to pronephric brush border defects and loss of cilia dynein arms. Ultrastructure of WT (3.5 dpf) pronephric duct showed dense brush border of apical microvilli extending from the epithelial cells and the presence of numerous cilia in the lumen (A). Apical microvilli were absent in dyx1c1 morphants (ATGMO+SPMO) at 3.5 dpf (B). Arrow in blue denotes missing brush border and blue arrowhead denotes cilia. Electron micrography of pronephric cilia axoneme in dyx1c1 morphant embryo at 3.5 dpf showed the absence of both ODA and IDA on the outer microtubules (D) whereas the dynein arms were present in WT embryos (C). Coinjection of dyx1c1 mRNA with MO rescued both ODA and IDA (E). Arrows in red indicate ODA and IDA in WT, morphant and rescued embryo. Scale bars in panels A and B indicate 1 μm and 2 μm respectively. Scale bars in panels C and D are 100 nm and 200 nm in E. PHENOTYPE:
|
|
Morphological phenotypes induced by ATGMO and SPMO. Both ATGMO and SPMO when injected alone produced identical phenotypes. Hydrocephalus and kidney cysts were clearly visible in the morphants at 2 dpf (B & C) and 3 dpf (E & F) respectively as compared to the nomal phenotype in wild-type (A & D). Arrows denote hydrocephalus and kidney cysts are denoted by arrowheads. RT-PCR showing the efficiency of SPMO at 3.5 dpf in embryos showing strong and weak phenotypes (G). Scale bars indicate 100 μm. PHENOTYPE:
|
|
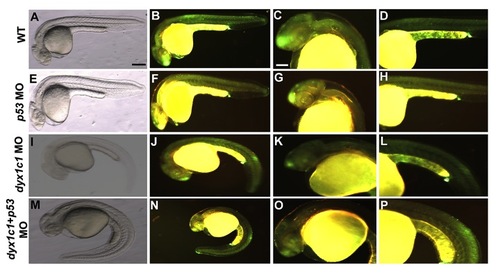
Morpholino specificity confirmed by coinjection with p53. Analysis of apoptotic cell death in WT (A–D), p53 morphants (E–H), dyx1c1 morphants (ATGMO+SPMO; I–L) and dyx1c1+ p53 morphants (M–P). Bright field (A,E,I,M) and fluorescent images of wild-type and morphants (B–D, F–H, J–L, N–P) at 1 dpf. Fluorescent signal in dyx1c1 morphants appeared similar to that seen in WT. dyx1c1 morphant phenotype was not affected by p53 coknockdown. Scale bars indicate 100 μm. |
|
Dynein arms of olfactory cilia affected in dyx1c1 morphants. Ultrastructure of olfactory cilia at 3 dpf showed loss of ODA and IDA in dyx1c1 morphants (B) as compared to WT (A). Coinjection with dyx1c1 mRNA rescued both the dynein arms (C). Arrows denote ODA and IDA. Scale bars indicate 200 nm. PHENOTYPE:
|
|
dyx1c1 mRNA rescues cilia defects in dyx1c1 morphants. Anti-acetylated tubulin staining of cilia in the pronephros of wild-type (A), dyx1c1 morphant (B) and mRNA coinjected embryo (C). Percentage of embryos showing left-side (normal), right-side (situs inversus) placement of liver and heterotaxia in wild-type, dyx1c1 morphants and mRNA injected embryos (D). Scale bar indicate 10 μm. |

Unillustrated author statements PHENOTYPE:
|