- Title
-
beta-arrestin control of late endosomal sorting facilitates decoy receptor function and chemokine gradient formation
- Authors
- Mahabaleshwar, H., Tarbashevich, K., Nowak, M., Brand, M., and Raz, E.
- Source
- Full text @ Development
|
Expression patterns of β-arrestin genes, cxcl12a and cxcr7b and control of Cxcr7b localization by β-arrestins. (A-E) Expression pattern of arrdc1 (A), arrb2a (B), arrb2b (C), cxcl12a (D) and cxcr7b (E) in 12-hpf zebrafish embryos. (F) Partial overlap of arrb1a, arrb2b, cxcr7b and cxcl12a expression patterns in the anterior somites, freeing lateral Cxcl12a expression domains (magenta) from Cxcr7 and β-arrestin effects. (G) The procedure for generating uniform Cxcr7b-EGFP expression (green) and cell clones knocked down for β-arrestins by injection with MOs targeting all β-arrestins (or control MO) and Histone H2B-mCherry for clone labeling (red). (H) In control 7-hpf mosaic embryo (left), Cxcr7b is found in small vesicles. In cells compromised for β-arrestin function (red nuclei, right), Cxcr7b vesicles are often enlarged. (I) Average colocalization coefficient (see supplementary material Fig. S12) among Cxcr7b-ECFP and Rab7-mCherry (at 5 hpf), Lamp1-DsRedmonomer and Rab5-mCherry (at 8 hpf) in control and β-arrestin knockdown cells. Data were averaged among six measurements (one image per embryo, <80 cells in the field of view). *P<0.05, **P<0.01, Student’s t-test. Error bars indicate s.e.m. EXPRESSION / LABELING:
PHENOTYPE:
|
|
β-arrestins influence trafficking of Cxcr7b into and out of Lamp1-positive endosomes. (A) Snapshots from supplementary material Movie 1 (example 1) showing that a Cxcr7-EGFP-labeled vesicle enters (arrow, upper panels) and splits from (arrow, lower panels) the Lamp1-DsRedmonomer-labeled endosomes in control cells. Time stamps indicate minutes:seconds. First frame corresponds to 1:33 (upper left) and 2:36 (lower left) in supplementary material Movie 1. (B) In cells lacking β-arrestin, Cxcr7-EGFP-labeled vesicles fuse with Lamp1-DsRedmonomer vesicles. First time point corresponds to 2:54 in supplementary material Movie 2. Arrow marks the vesicle of interest. (C) Illustration of a transplantation experiment. Images illustrate decay of the Cxcl12a-EGFP signal, relative to that of the receptor (supplementary material Movie 3). Arrow points at the vesicle of interest. (D) Representation of the internalization and subsequent recycling of Cxcr7b to the cell membrane after targeting Cxcl12a to degradation (left). In the absence of β-arrestin, Cxcr7b accumulates in late endosomes, compromising sink function (right). PHENOTYPE:
|
|
β-arrestins control the level of Cxcl12a in the tissue and can direct PGC migration. (A) In embryos depleted for endogenous Cxcl12a and provided with uniform Cxcl12a (injection of MO-resistant cxcl12a mRNA), germ cells accumulate in regions depleted of β-arrestin (red). Such primordial germ cell (PGC) localization is not observed in clones in which β-arrestin was not affected and depends on Cxcl12a and Cxcr7b function. The percentage of PGCs located in each domain in 11-hpf embryos was determined and an average among the embryos (N) for each treatment was calculated. Error bars indicate s.e.m. A significant difference (P<0.001, Student’s t-test) was observed for the experiment shown on the left. (B) A typical result for the experiments shown in A, in which β-arrestin activity is similar in regions A and B (control MO) or is knocked down in region B (β-arrestin MOs). (C) Generation of clones depleted of β-arrestin (or control clones) in embryos uniformly expressing CyPet (a cyan fluorescent protein derivative), Cxcr7b and Cxcl12-Venus. (D) Intensity profiles of Cxcl12a-Venus in control chimera (top) and β-arrestin morphant chimera (bottom) measured from the embryos presented in C at 11 hpf. a.u., arbitrary units. |
|
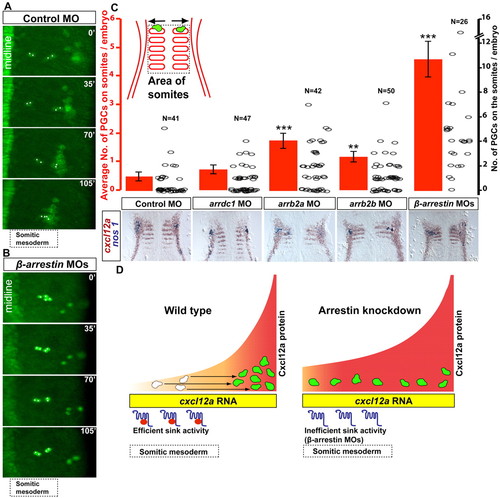
Role of β-arrestins in discrimination between Cxcl12a expression domains. (A,B) Snapshots from supplementary material Movie 5 (A) and Fig. S6 (B). In early somitogenesis stages, PGCs (asterisks) specified close to the midline migrate laterally (A). In an embryo lacking β-arrestin function (B), this movement is not observed. (C) β-arrestins promote migration of PGCs away from the somites. Red bars (y-axis on left) indicate the average number of cells remaining in the somites; error bars indicate s.e.m. The distribution of the results is indicated by circles (y-axis on right); N, the number of embryos analyzed; **P<0.05, ***P<0.001, Student’s t-test. Bottom panels show examples of in situ hybridizations using nanos1 (nos1; blue, PGCs) and cxcl12a (brown) probes used to generate the graphs. (D) A model for PGC migration during early somitogenesis in wild type (left) and in embryos compromised for β-arrestins (right). The sink function of Cxcr7b (purple), aided by β-arrestins (red ellipsoid), converts uniform cxcl12a mRNA expression into a chemotactic Cxcl12a gradient, directing the PGCs to the right. In the absence of β-arrestins, Cxcr7b function is compromised, increasing Cxcl12a levels in the forming somites and hindering the migration of PGCs towards their target. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Expression pattern of β-arrestins during early zebrafish development. (A) RT-PCR analysis shows that arrdc1, arrb2a and arrb2b are expressed during developmental stages when PGCs actively migrate (8 hpf), with a control reaction representing the constitutively expressed ornithine decarboxylase 1 (odc1) mRNA. (B-J) RNA expression patterns of arrdc1, arrb2a and arrb2b at the indicated developmental stages. |
|
Cxcr7b localization to the plasma membrane is not enhanced by β-arrestin knockdown. Wild-type embryos were co-injected at the 1-cell stage with mRNAs for Cxcr7b-EGFP-globin32UTR and mCherry-F-globin32UTR as well as with MO mixes containing either control MO (COMO) or β-arrestin MOs. Confocal images (63×) were acquired at (A) 50% epiboly (5.5. hpf) and (B) 80% epiboly (8 hpf). The graph shows the quantification of the pixel intensity of Cxcr7b-EGFP located on the cell membrane at 5.5 hpf. The average pixel intensity in the control is set to 1. N, number of cells analyzed; **P<0.05, Student’s t-test. |
|
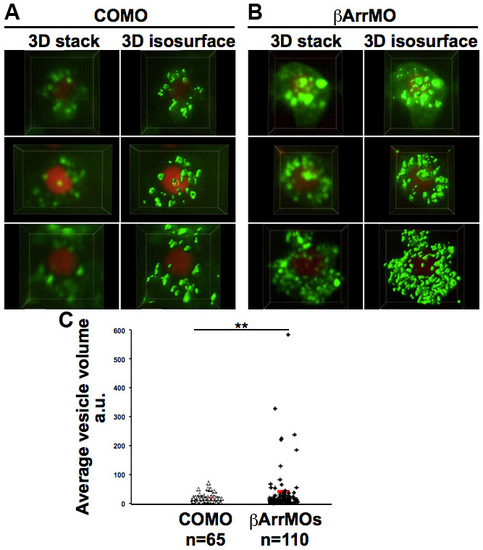
Difference in size of Cxcr7b foci in control cells and cells knocked down for β-arrestin function. (A,B) Original 3D stacks and their 3D surface rendering (3D isosurface) performed using Imaris software on six different cells. Embryos were injected with EGFP-Cxcr7b and H2B-mCherry mRNAs and either control (A) or β-arrestin (B) MOs; cells were imaged at 8-9 hpf. (C) The average size of vesicles in the cells shown in A and B. **P<0.05, Student’s t-test; n, number of vesicles analyzed. |
|
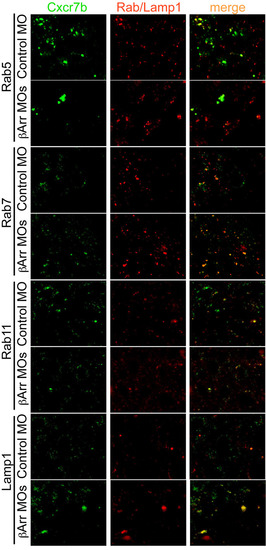
Representative images of Cxcr7b localization relative to Rab5, Rab7, Rab11 or Lamp1-positive endosomal compartments in control and β-arrestin knockdown cells. Images were captured at 5 hpf for Cxcr7b/Rab7 colocalization and at 8-9 hpf for colocalization with the other endosomal markers. |
|
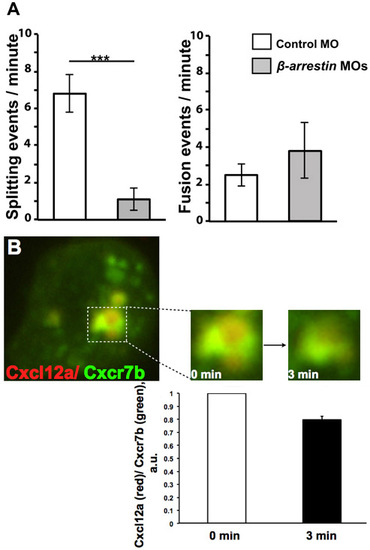
Quantitation of splitting and fusion events of Cxcr7-containing vesicles and decay of Cxcl12-Cherry signal with respect to that of Cxcr7b-EGFP. (A) Cxcr7b foci in wild-type control cells show higher numbers of splitting events per minute than cells knocked down for β-arrestin function (***P<0.001, Student’s t-test). By contrast, no significant difference in fusion of Cxcr7b foci was observed between control and β-arrestin knockdown cells. Error bars indicate s.e.m. Analysis was performed on six time-lapse movies. (B) Dynamics of Cxcl12a degradation in β-arrestin morphant cells. Graph illustrates the intensity ratio between Cxcl12a (tagged with mCherry, red) and Cxcr7b (EGFP-tagged, green) channels measured for individual foci at two time points, 3 minutes apart. Analysis was performed for three foci from two time-lapse movies with the ratio at the beginning of the movie (0 min) set to 1 for each of the foci. Error bars indicate s.e.m. |
|
The effect of β-arrestin knockdown on Cxcr7b level. Western blot analysis showing the level of Cxcr7b-EGFP and α-tubulin (loading control) in whole embryo lysates at 11 hpf. Embryos were injected at 1-cell stage with Cxcr7b-EGFP mRNA and control (COMO) or β-arrestin MO (βArrMOs). Cxcr7b-EGFP protein level was detected using anti-GFP antibody (Clontech, 632377). Endogenous α-tubulin was detected using anti-tubulin antibody (Acris, C0379). Graph represents the ratios between Cxcr7b and α-tubulin band intensities as determined by 2D densitometry (AIDA, BioImaging). |
|
The effect of Cxcr7b on Erk1/2 phosphorylation. Western blot analysis showing the level of pErk1/2, total Erk1/2 and α-tubulin (loading control) in whole embryo lysates at 10-11 hpf. (A) The levels of Erk1/2 and its phosphorylated form are not altered by knocking down Cxcr7b (cxcr7b MO, compared with control MO), nor by overexpressing the protein (cxcr7b RNA, compared with gfp RNA). (B) The level of phosphorylated Erk1/2 is significantly reduced by treatment with 100 μM Mek1/2 inhibitor U0126. |
|
β-arrestin knockdown does not affect the subcellular distribution of Cxcr4b and the observed β-arrestin-induced phenotypes do not result from β-arrestin-dependent Cxcr4b internalization. (A) Wild-type embryos were co-injected at 1-cell stage with WT-Cxcr4b-DsRed-globin32UTR RNA or with mut-Cxcr4b-DsRed-globin32UTR mRNA encoding a mutated version of Cxcr4b that is non-internalizable (Minina et al., 2007) and either control or β-arrestin MO mix. Confocal scans (63×) were performed at 8 hpf. (B) ody/ (cxcr4b) embryos lacking a functional Cxcr4b receptor were co-injected at 1-cell stage with control mRNA (EGFP-F-nos32UTR) or with the mut-Cxcr4bEGFP-nos32UTR mRNA and either with control MO or β-arrestin MO mix. Embryos were fixed at 11-12 hpf and subjected to double-color whole-mount in situ hybridization for nanos (blue) and Cxcl12a (red). PGCs positioned on the somites were counted and averaged for 20 embryos per experimental group (N). Error bars indicate s.e.m. **P<0.05, ***P<0.001, Student’s t-test. The percentage of PGCs residing on the somites is presented, showing that knockdown of β-arrestins enhances the localization on the somites of PGCs guided by a non-internalizable Cxcr4b protein. (C) Wild-type embryos were co-injected at 1-cell stage with EGFP-F-nos32UTR mRNA and WT-Cxcr4b-DsRed-nos32UTR and either control or β-arrestin MO mix, showing no effect of β-arrestin knockdown on Cxcr4b membrane localization in PGCs. Confocal images (63×) were captured at 8 hpf. (D) ody/ embryos were co-injected at 1-cell stage with mut-Cxcr4bEGFP-nos32UTR mRNA and either control MO or β-arrestin MO mix showing no effect of β-arrestin on the localization of the non-internalizable Cxcr4b in the PGCs. Confocal images (63×) were captured at 8 hpf. |
|
Effect of β-arrestin knockdown on Cxcl12a distribution is Cxcr7b dependent. (A) Scheme for generating clones depleted of β-arrestin (similar to that in Fig. 3C). Such clones show a higher level of Cxcl12-Venus (lower panels). (B) In a similar experiment in which Cxcr7b is uniformly knocked down (upper scheme), clones knocked down for β-arrestin do not exhibit the increase in Cxcl12a-Venus (lower panels). Imaging was performed at 11 hpf. |
|
Efficiency of arrb2a and arrb2b MOs. (A) DIC and epifluorescence images of 4.5-hpf embryos co-injected with MO-sensitive arrb2a-EGFP-globin mRNA, mCherry-F-globin mRNA and either non-targeting control MO (upper panels) or arrb2a MO (lower panels). (B) DIC and epifluorescence images of 4.5-hpf embryos co-injected with MO-sensitive arrb2b-EGFP-globin mRNA, mCherry-F-globin mRNA and either non-targeting control MO (upper panels) or arrb2b MO (lower panels). |
|
Quantification of colocalization of Cxcr7b with endosomal markers. (A) Representative images of cells co-expressing Cxcr7b and endosomal markers, Rab7 in this example, analyzed using the Zeiss ZEN colocalization module. (B) Colocalization scatter plot of gray intensity values for each pixel in ECFP (Cxcr7) and mCherry (Rab proteins)/DsRed (Lamp1) channels (1 and 2 represent ‘non-colocalizing’ pixels of each channel, 3 represents colocalization intensities). The intensity colocalization coefficient determined from such graphs was averaged among six independent measurements (1 measurement per embryo). |