- Title
-
Pathfinding in a large vertebrate axon tract: isotypic interactions guide retinotectal axons at multiple choice points
- Authors
- Pittman, A.J., Law, M.Y., and Chien, C.B.
- Source
- Full text @ Development
|
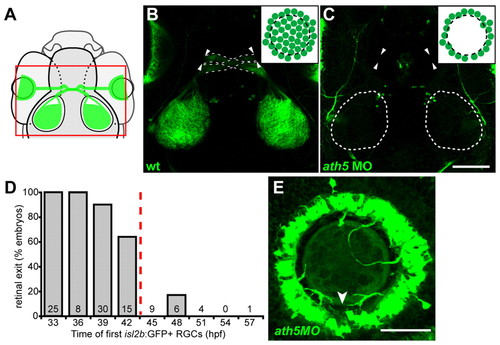
ath5 MO blocks differentiation of early- but not late-born RGCs. (A) Normally, early RGCs differentiate in a wave across central retina (blue arrow); late RGCs are then added centrifugally (red arrows). (B,C) 6 dpf isl2b:GFP, lateral views, anterior towards the left. Insets schematize cell bodies, axons and optic nerve head (star). (B) Wild-type eye shows GFP+ RGCs throughout central retina; axons are obscured by cell bodies. (C) A high dose of ath5MO blocks differentiation of early RGCs, but late RGCs still form (arrows). Without central RGCs, peripheral axons are visible (arrowheads). (D) Dose-response curve showing timing of RGC formation with different doses of ath5MO. In wild type and with 3 ng control MO, GFP+ RGCs appear by 33 hpf. Increasing concentrations of ath5MO increasingly delay the appearance of the first RGCs. A, anterior; D, dorsal; P, posterior; V, ventral. Scale bar: 50 μm. |
|
Early RGCs are necessary for axons to exit eye. (A) Diagram showing field of view for B,C. (B,C) 5 dpf isl2b:GFP, confocal z-projections; dorsal views, anterior upwards. Insets show cell bodies in eye. (B) Wild-type axons exit eye (arrowheads), project through the optic chiasm (broken lines) and to the optic tecta. (C) With high dose of ath5 MO, axons do not exit eyes (arrowheads) or innervate tecta (outlined). (D) Retinal exit in dose-response experiment of Fig. 1D, plotting percentage of embryos in which axons exit the eye against the time at which their first isl2b:gfp-positive RGCs were born. When RGCs are born by 42 hpf, axons usually exit the eye; when delayed after 42 hpf, axons rarely exit. Number of embryos is indicated at base of each bar. (E) 72 hpf isl2b:GFP, confocal z-projection; dorsal upwards. In a high-dose ath5 morphant, axons from peripheral RGCs remain trapped in the RGC layer without entering the optic nerve. To better appreciate 3D structure, see volume reconstruction in Movie 1 in the supplementary material. Arrowhead indicates the optic nerve head, stained by anti-Pax2 (not shown). Scale bars: 100 μm in C; 50 μm in E. EXPRESSION / LABELING:
PHENOTYPE:
|
|
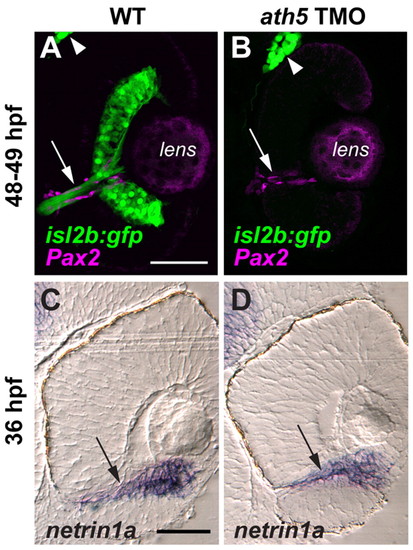
ath5 MO does not appear to affect the molecular and cellular environment of the optic nerve head or the intraretinal optic nerve. Coronal sections through 48-49 hpf isl2b:GFP (A,B) and 36 hpf non-transgenic (C,D) eyes, from either uninjected embryos (A,C) or embryos injected with high dose ath5 TMO (B,D). (A,B) Pax2a antibody staining (magenta) labels presumptive glial cells which line the intraretinal region of the optic nerve (arrows) in both wild type (A) and ath5 morphants (B). RGCs and their axons (green) are present in wild type (A), but not in ath5 morphants (B). The trigeminal ganglion (arrowheads) is also labeled by isl2b:gfp and serves as a staining control. (C,D) In situ hybridization shows that netrin1a expression (arrows) surrounds the optic nerve and optic nerve head in wild type (C), and is unchanged in ath5 morphants (D). Scale bars: 50 μm. |
|
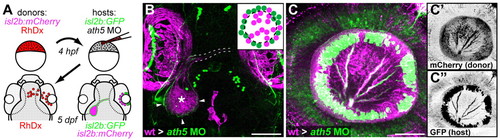
Transplanted WT central RGCs rescue retinal exit in ath5 morphants. (A) Blastula transplants from isl2b:mCherry donors resupply ath5 morphant hosts, labeled with isl2b:gfp, with early RGCs. RhDx, rhodamine-dextran cell lineage marker. (B) Resupplied WT RGCs and axons (magenta) are sufficient to rescue host axons in morphants (green). Pigment cell autofluorescence is seen around eyes and at dorsal midline (magenta). Retinal axons project across chiasm (broken lines); donor axons terminate in central tectum (asterisk), while host axons terminate in peripheral tectum (arrowheads). Dorsal view, 5 dpf. (C) Lateral view of 5 dpf eye showing peripheral host axons (green) fasciculating with resupplied WT axons (magenta). (C′) Donor and (C″) host axons, reverse contrast. Scale bars: 100 μm in B; 50 μm in C. |
|
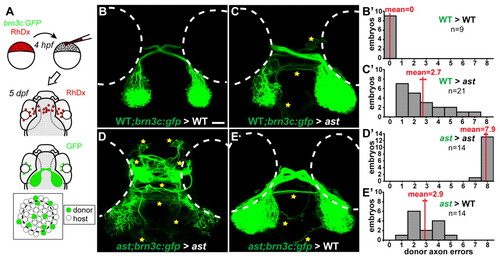
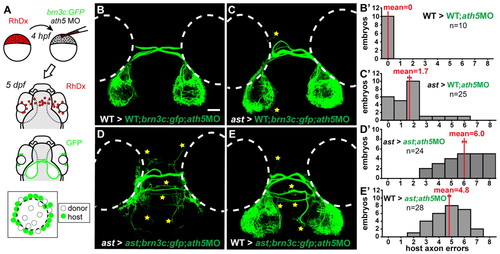
Axon-axon interactions strongly influence retinotectal pathfinding. (A) Transplants yield GFP-expressing donor RGCs in host eyes. (B-E) Dorsal views, 5 dpf, rostral upwards. Donor axons labeled with brn3c:GFP; pathfinding errors indicated by yellow stars. (B′-E′) Error quantitation. (B,B′) In wild-type> wild-type transplants, donor axons pathfind perfectly. (C,C′) By contrast, when transplanted into ast hosts, wild-type axons make significantly more errors. (D,D′) As expected, ast donor axons make many errors in ast hosts. (E,E′) However, when transplanted into wild-type hosts, ast axons make significantly fewer errors. Scale bar: 50 μm. |
|
Pioneer axons influence pathfinding of follower axons. (A) Transplants resupply early RGCs to brn3c:GFP hosts injected with ath5 MO. (B-E) Dorsal views at 5 dpf, rostral upwards. Host axons terminate peripherally on the tectum; errors indicated by stars. (B′-E′) Error quantitation. (B,B′) In wild type>wild type;ath5 MO transplants, host follower axons pathfind normally. (C,C′) When pioneers are replaced with ast cells, wild type follower axons make significantly more mistakes. (D,D′) In ast>ast;ath5MO transplants, host follower axons make many errors. (E,E′) When pioneers are replaced with wild-type cells, follower ast axons make fewer errors but are not completely rescued. Scale bar: 50 μm. |
|
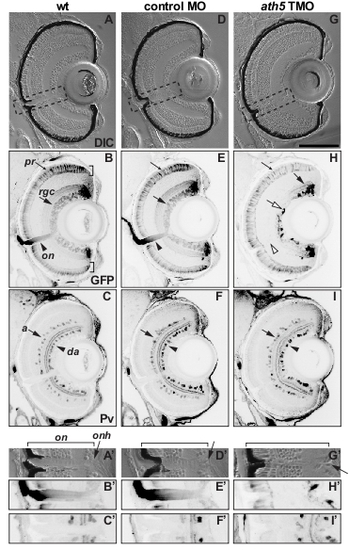
ath5 MO does not grossly affect the lamination, position of presumptive optic nerve or neurogenesis of non-RGCs in the retina. Coronal sections through 6 dpf isl2b:GFP eyes after double-staining for GFP (B,E,H) and parvalbumin (Pv; C,F,I). (A-C) Uninjected wild type; (D-F) larva injected with control MO; (G-I) larva injected with high dose of ath5 TMO. (A′-I′) High-power views of A-I (see boxed areas in A,D,G). (A,D,G) DIC images show that retinal lamination is not affected in ath5 morphants, with the exception of a severely reduced RGC layer. (A′,D′,G′) The location of the presumptive optic nerve (on) and optic nerve head (onh, arrows), as well as a break in the RPE that represents the normal exit point for retinal axons, are clearly identifiable in ath5 morphants. (B,E,H) In controls, isl2b:GFP staining shows RGCs throughout the entire RGC layer (rgc, arrows) and their axons exiting the eye in the optic nerve (on, arrowhead). However, in ath5 morphants (H), central RGCs do not differentiate, although later-born RGCs in the periphery differentiate normally (arrow). In addition, no axons exit the eye (open arrowhead). Staining in central retina represents axons from peripheral RGCs (open arrow). GFP+ photoreceptors labeled by isl2b:gfp differentiate normally in ath5 morphants (B,E,H; small arrows). (C,F,I) Differentiation of Pv+ amacrine cells in the inner nuclear layer (a, arrows) and displaced amacrine cells in the RGC layer (da, arrowheads) appears unaffected in ath5 morphants. Scale bar: 100 μm. |
|
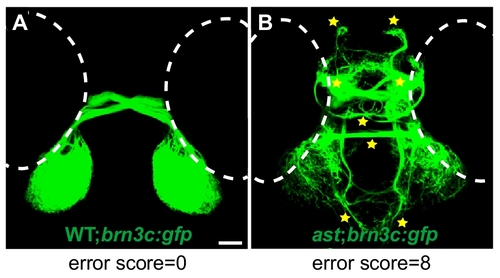
Eight-point scoring system used to quantify retinal axon errors. Dorsal views of retinotectal projection of 5dpf isl2b:gfp wild type and ast larvae. (A) In wild-type embryos, retinal axons always pathfind to the tectum, and errors are almost never seen. (B) In ast-null mutants, eight classes of errors are commonly seen, including midline crossing at the habenular and posterior commissures, and projections to the left and right telencephalon, diencephalon and ventral hindbrain (stars). Counting the classes of errors made by misguided axons in each larva yields an error score between 0 and 8. |
|
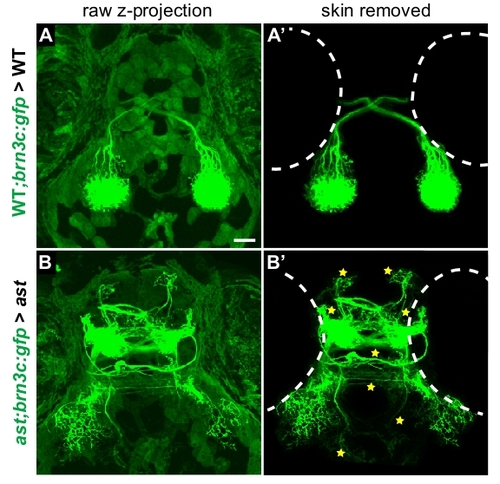
Background removal procedure used for transplant images. Dorsal views of projections made by donor axons in 5 dpf host larvae, control transplants. (A,B) Maximum-intensity z-projections of unedited confocal stack in wild type>wild type (A) and ast>ast (B) transplants. Autofluorescence in skin, superficial to axons, partially obscures the retinal projection. (A′,B′) Confocal projections shown after removing autofluorescence slice-by-slice by manual editing in ImageJ and Adobe Photoshop. Retinal axons are unaffected, but can be seen more clearly in the absence of skin fluorescence. Stars indicate errors (always counted in unedited confocal stacks); dotted outlines show eye positions. All transplant images in Figs 5 and 6 were processed in this manner. |