- Title
-
TNF{alpha}-dependent hepatic steatosis and liver degeneration caused by mutation of zebrafish s-adenosylhomocysteine hydrolase
- Authors
- Matthews, R.P., Lorent, K., Mañoral-Mobias, R., Huang, Y., Gong, W., Murray, I.V., Blair, I.A., and Pack, M.
- Source
- Full text @ Development
|
Hepatic steatosis and liver degeneration in dtp zebrafish larvae. (A-D) Lateral views of 5-dpf wild-type (wt, A,C) and dtp mutant (B,D) larvae showing small liver (outlined, arrow) and reduced yolk consumption (y) in dtp. (C,D) RNA in situ hybridization for the liver marker transferrin (tfa) demonstrates smaller liver size (arrow) in dtp. (E-J) Liver histology showing irregular dtp hepatoctye nuclei beginning at 3 dpf (compare F with E) and with cellular degeneration that is pronounced at 5 dpf (compare J with I). (K,L) Oil Red O (ORO) staining reveals neutral lipid in the small liver of a 5-dpf dtp larva (L) but not in the wild type (K). (M-P) Electron micrographs of the enlarged nuclei (compare N with M, arrowheads), mitochondrial defects (compare P with O, m) and lipid droplets (P, arrows) in 5-dpf dtp hepatocytes. c, canaliculus; eso,esophagus; liv, liver. |
|
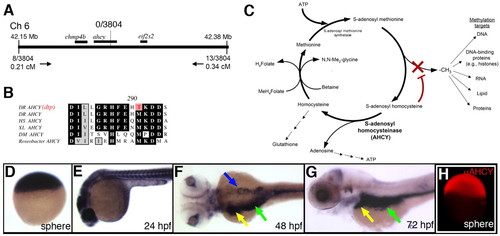
Identification of ahcy as the dtp gene. (A) Schematic of the zebrafish dtp locus. The nearest polymorphic markers are indicated, with corresponding numbers of recombinants and calculated genetic distances. (B) Deduced amino acid sequence near the ahcy mutation identified in dtp larvae (indicated in red; DR, Danio rerio), with corresponding amino acid sequence of orthologous Ahcy proteins from Homo sapiens (HS), Xenopus laevis (XL), Drosophila melanogaster (DM) and Roseobacter. (C) Schematic of the methionine metabolism pathway. Reduced Ahcy activity is predicted to increase levels of SAH, which inhibits methyltransferases. (D-G) ahcy expression in staged zebrafish embryos and larvae. Ubiquitous expression is evident through 24 hpf. Expression at 48 hpf and 72 hpf is restricted to the liver (yellow arrow), intestine (green arrow) and pancreas (blue arrow). (H) Immunostaining at sphere stage, showing ubiquitous immunoreactive Ahcy protein. EXPRESSION / LABELING:
|
|
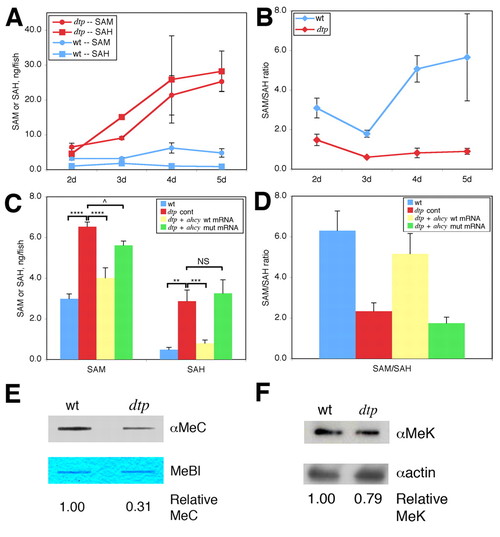
Altered methionine and methyl metabolism in dtp larvae. (A) SAM and SAH levels, and (B) SAM:SAH ratio in wild-type (wt) and dtp zebrafish larvae. (C) SAM and SAH levels in 2-dpf wild-type and dtp embryos injected with control morpholino (dtp cont), and dtp embryos injected with either wild-type ahcy mRNA or mRNA carrying the ahcydtp missense mutation. ****P<0.00001, ***P<0.0001, **P<0.01, ^P<0.05; NS, not significant (P=0.3). (D) Calculated SAM:SAH ratio for the four conditions in C. Differences in the SAM:SAH ratio between B and D arise from experimental variation. (E) Anti-methylcytosine (αMeC) slot blot of genomic DNA derived from wild-type or dtp larvae, with Methylene Blue counterstain (MeBl). (F) Western blot showing reduced levels of methylated lysine residues (αMeK) in protein from dtp larvae; actin provided a loading control (αactin). |
|
Increased expression of lipogenic and stress-responsive genes in dtp larvae, and rescue of dtp steatosis and liver degeneration with tnfa knockdown. (A-D) Expression levels of peroxisome proliferator-activated receptor gamma (pparg, A), sterol response element binding protein 1 (srebp1, B), glutathione peroxidase (gpx, C) and tumor necrosis factor alpha (tnfa, D) in 4-dpf dtp versus wild-type (wt) siblings. Inset for D shows Tnfα western blot in 5-dpf dtp larvae. *P<0.05, **P<0.005; NS, not significant (P=0.07). (E,F) Whole-mount RNA in situ hybridization showing enhanced tnfa expression in the liver (outlined), intestine (arrow) and swim bladder (*) of a dtp larva (F) as compared with a wild-type sibling (E). (G) Western blot showing reduced Tnfα protein in tnfa morpholino-injected larvae. (H) Lateral view of a fixed 5-dpf dtp larva injected with tnfa antisense morpholino oligonucleotide at 2 dpf; liver size is enlarged (outlined), compare with live dtp 5-dpf larva depicted in Fig. 1. (I) Liver rescue in tnfa morpholino-injected dtp mutant as revealed by transferrin (tfa) in situ hybridization. (J,K) tnfa knockdown also rescues dtp liver histology (compare with Fig. 1J). (L) Mitochondrial ultrastructure is improved and steatosis is rescued by tnfa knockdown. eso, esophagus; li, liver; m, mitochondria. EXPRESSION / LABELING:
|
|
Ahcy inhibition in adult zebrafish causes liver steatosis and activates tnfa expression. (A) Quantitative real-time PCR reveals elevated tnfa expression in adult zebrafish liver following 7-day treatment with the Ahcy inhibitor 3-deaza-adenosine (deazaA). (B,C) Oil Red O staining reveals steatosis in deazaA-treated adult livers, as compared with control. (D) Reduced liver mitochondrial glutathione (GSH) in deazaA-treated adult fish compared with control. *P<0.05, **P<0.005; error bars indicate s.e.m. |
|
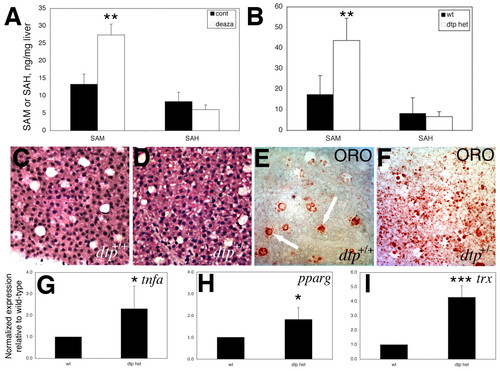
Liver steatosis, increased lipogenic gene expression, and methionine metabolism defects in adult dtp heterozygotes. (A,B) SAM and SAH levels from livers of (A) adult fish treated with deazaA and (B) adult dtp heterozygotes, as compared with wild-type (wt) controls. (C,D) Liver histology from adult wild-type and heterozygous dtp fish showing vesicular clearing consistent with steatosis. (E,F) Oil Red O staining of adult wild-type and heterozygous dtp fish reveals steatosis in the heterozygotes. Lipid is evident in wild-type liver sinusoids (E, arrows), but not in hepatocytes. (G-I) Elevated expression of tnfa (G), pparg (H) and the ROS-sensitive gene thioredoxin reductase (trx) (I), in the liver of adult dtp heterozygous versus wild-type fish. *P=0.05, **P<0.05, ***P<0.005. |
|
Ahcy morpholino injection leads to hepatic steatosis. Oil Red O (ORO) staining of 5-dpf larvae injected with control (A) or antisense morpholino directed against the ahcy AUG site (B). Injections were performed at 2 dpf. Note the diffuse ORO staining of the liver (outlined) in B, which is not present in the control. Injection with a splice-blocking morpholino had a similar effect. PHENOTYPE:
|
|
Persistent liver phenotype and increased tnfa expression in gnotobiotic dtp larvae. (A,B) Left lateral view of transferrin (tfa) in situ hybridizations shows reduced liver size in gnotobiotic dtp versus wild-type larvae. (C) tnfa expression remains elevated in gnotobiotic dtp larvae. (D,E) Tryptic soy agar plates plated with germ-free medium (D) or standard medium (E). |
|
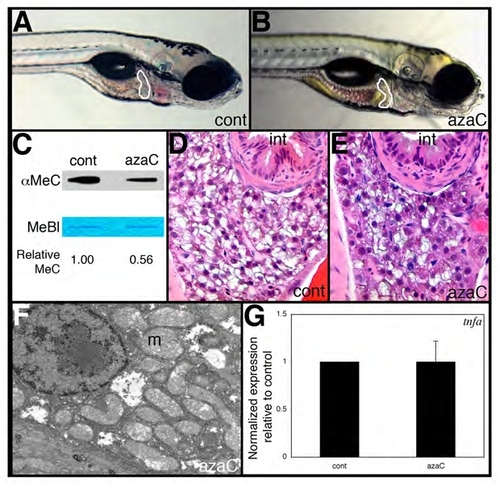
5-Azacytidine treatment does not phenocopy dtp liver defects. (A,B) Lateral views of 5-dpf wild-type larvae injected with azacytidine (B) or vehicle (control) at 2, 3 and 4 dpf. (C) Reduced DNA methylation in azacytidine-injected versus control larvae as revealed by anti-methylcytosine (αMeC) slot blot of genomic DNA, with Methylene Blue counterstain (MeBl). (D,E) Comparable liver histology in 5-dpf control and azacytidine-injected larvae. (F) Liver electron micrograph from an azacytidine-injected larva shows normal mitochondrial ultrastructure and no evidence of steatosis. (G) tnfa expression is not elevated in azacytidine-injected larvae. |