- Title
-
Disruption of Esrom and Ryk identifies the roof plate boundary as an intermediate target for commissure formation
- Authors
- Hendricks, M., Mathuru, A.S., Wang, H., Silander, O., Kee, M.Z., and Jesuthasan, S.
- Source
- Full text @ Mol. Cell Neurosci.
|
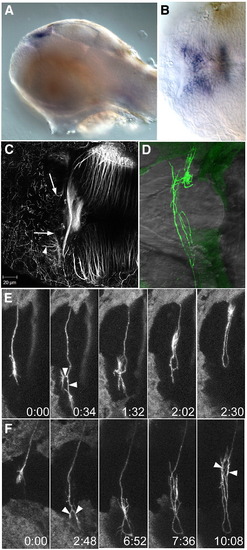
Habenular commissural axons pause before and after crossing. (A–D) Time series of HC development in the Kaede line (see Supplementary Movie 1). (A) Commissural axons enter the habenulae as a bundle but splay out near the medial boundary of the habenula (arrows). (B) A single pioneer axon crosses (arrowhead) while other axons remain outside the roof plate. (C) As several more axons cross the midline, the bundle within the habenulae thickens, and more axons accumulate at the medial boundary. This is visible here in the left habenula (arrowhead); the right habenula is obscured by a melanophore (m). (D) As the commissure thickens, axons tips are still visible outside the roof plate (arrows). (E–H) A 2-day-old embryo, in which a subset of HC axons from the left EmT have been labeled with the lipophilic tracer DiD. (E, F) Several axons prior to crossing, with their tips within the left habenula. Two large growth cones extending multiple filopodia can be seen (arrowhead and arrow). (G) One axon has begun crossing ahead of the others (arrow), while the growth cone indicated with an arrowhead remains ipsilateral. (H) The first axon (arrow) has reached the contralateral habenula and shows a more complex morphology than during crossing, a second axon has almost finished traversing the roof plate, the third axon (arrowhead) has still not crossed. (I) An embryo unilaterally electroporated with HuC:Gal4/UAS:EGFP with commissural axons in a bundle which has reached the contralateral habenula boundary (arrow). (J) In another embryo at a slightly later stage, a few axons have entered the contralateral habenula (arrowhead) while others remain outside the roof plate (arrow). (K–N) A time series of a 2 dpf EGFP-electroporated embryo shows axons pausing ipsi- and contralaterally, but fasciculated and rapidly extending within the roof plate (N, see Supplementary Movie 2). Three growth cones indicated in panel M were tracked over time and their distance from the midline plotted (O, arrowhead color corresponds to line color). The axons extend most rapidly around the midline; dotted line in (O) indicates the time point shown in (M). Dashed line indicates the midline. Time is h:min. Scale bars = 20 μm (A–D), 10 μm (E–H), and 25 μm (I–N). Anterior to the left. m, melanophore; P, pineal organ; pc, posterior commissure. |
|
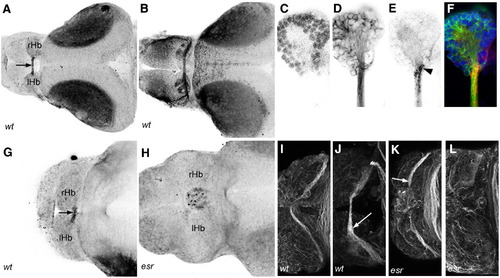
The habenular commissure is absent in esrom mutants. (A) Axon tracts in the dorsal forebrain of 3 dpf wt embryo, visualized with an antibody to acetylated tubulin. The habenular commissure (arrow) is visible between the left and right habenulae. (B) An esrom mutant at 3 dpf. The habenular commissure is absent. The posterior commissure is indicated (arrowhead). (C–F) Unilateral electroporation with eGFP, showing the presence of the habenular commissure (arrow, C) and contralateral terminations (arrowheads, E) in a wild type embryo. These are absent in an esrom mutant (D, F). Habenular afferents can be visualized with an antibody to mGluR2/3 (G, H) or SV2 (I, J). The commissure is visible in wild types (arrow, G, I). In mutants (H, J), axons terminate in the ipsilateral neuropils (red arrowheads). All embryos are shown in dorsal view, except panels E and F which are lateral views. rHb: right habenula; lHb left habenula; TeO: tectum opticum. Scale bar = 20 μm. PHENOTYPE:
|
|
Mutant axons stall at the roof plate boundary. (A) Time series in an esrom;Kaede embryo shows axons oriented toward the midline and extending and retracting (arrows, see Supplementary Movie 3). After several hours, innervation can be seen developing in the ipsilateral neuropil (arrowhead). (B) Time series of a single mutant growth cone (arrow), maintaining a complex morphology and dynamic behavior for several hours (see Supplementary Movie 4). P: pineal. Scale bar = 20 μm. |
|
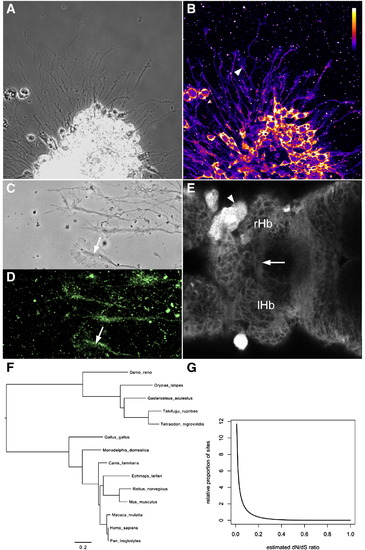
Esrom is a broadly expressed and slowly evolving protein. (A, B) Axons extending from cultured forebrain neurons. The 7014 antibody to Esrom labels all cell bodies and axons. The core (arrowhead) of growth cones is more strongly labeled than the periphery. (C, D) Cultured axons labeled with the 2a anti-PAM antibody, with strong label in the phase-dense region (arrows). (E) An optical section through a 3 dpf brain labeled with the 7014 antibody. Axons in the habenular commissure are indicated (arrow). A xanthophore (arrowhead) is brightly labeled, both in the cytoplasm and nucleus. (F) The phylogeny used for the analysis of codon evolution. (G) The estimated distribution of evolutionary rate variation across codon sites (measured as the dN/dS ratio). The vast majority of sites have very low dN/dS ratios, suggesting that most codon sites are subject to very strong negative (stabilizing) selection. EXPRESSION / LABELING:
|
|
Esrom regulates Tsc2 and 4E-BP1 in forebrain axons. (A) Mutations in three alleles affect the C-terminus of esrom. cDNA sequence in esrtp03 homozygous embryos includes intronic sequence after exon 62 due to a G to A mutation at the first position of intron 62 (arrow). This leads to two alternative transcripts, containing either all or part of intron 62, depending on the partial activity of a cryptic splice donor (causing double peaks). Either insertion causes a frameshift and premature stop codon in exon 63. This mutation eliminates an RsaI site, which can be used for genotyping. In esrtn207b embryos, a T to A mutation (arrow) causes a stop codon and creates an AluI site. The protein is predicted to be truncated at serine 3586. The esrty130 mutation, which causes a weaker phenotype, was previously characterized but mislabeled as esrtp03 (D’Souza et al., 2005). A cysteine required for disulfide bond formation in the LDLRA motif is changed to alanine due to a T to A mutation in exon 81. Domain structure of Esrom showing the positions of the three lesions described here. BB, b-box; BR, basic rich; LZ, leucine zipper; RHD, RCC1 homology; PHR, PAM, Highwire, RPM-1 repeats; FIL, filamin-like; cMBD, c-Myc binding; NLS, nuclear localization signal; bHLH, basic helix–loop–helix; LDLRA, low-density lipoprotein receptor type A motif; RF, RING finger; ZF, zinc finger. (B, C) Cultured forebrain explants double-labeled with anti-acetylated tubulin (red) and anti-Ser939 phospho-Tsc2 (green). Frequent enrichment of phospho-Tsc2 is evident in distal axons and growth cones of the mutant explant (arrowheads in C). (D–F) High magnification view of the distal end of a mutant axon, showing that phospho-Tsc2 levels (E) are increased in a pattern complementary to that of acetylated tubulin (D). (G) Average intensity of phospho-Tsc2 immunofluorescence in the growth cone of esrom (pink) and wild type (green) forebrain axons. The units represent relative intensity. Measurements are based on 20 mutant and 20 wild type clumps (Mann–Whitney p < 0.001). (H, I) Phospho-Tsc2 immunofluorescence of brains isolated from a wild type sibling (H) and esrom mutant (I). Higher level of staining is seen in axon tracts of mutants (arrow, I). Images are projections of confocal z-stacks. (J, K) In the habenula, axonal (SV2-colocalized) phospho-Tsc2, shown in pseudocolor, is elevated in mutants, especially medially (arrows). (L, M) Phospho-Tsc2 label in the habenula. In the mutant (M), intensely labeled bundles (arrow) and individual axons (arrowhead) can be seen extending toward the midline in the medial right habenula. These are not seen in the wild type (L). Anterior to the left, medial is down in (L, M). (N, O) Immunofluorescence for phospho-4E-BP1in forebrain axons of wild type (N) and esrom mutant (O). Stronger labeling is visible in the tips of mutant axons. (P) Average ± standard deviation of phospho-4E-BP1 intensity. Measurements are based on n = 10 wt and 10 mutant clumps; p = 0.008. |
|
EphB receptors are surface-localized at the roof plate boundary. (A) Ephrin-B2/Fc binds to HC axons only within the commissure (arrow) at 3 dpf. (B) Ephrin-A2/Fc affinity probe indicates surface localization of EphA receptors in the HC, the habenular neuropils, stria medullaris and tract of the habenular commissure at 3 dpf. (C–F) Higher magnification view of the right habenula labeled with the nuclear stain Syto-11 (C), acetylated tubulin (D) and Ephrin-B2/Fc (E) shows that EphB receptors are surface-localized at the medial boundary of the habenula (arrowhead). (G, H) Ephrin-B2/Fc binding is detected in a wild type (G) but not in a mutant (H) at 2 dpf. (I–L) Immunofluorescence with a pan EphB antibody at 2 (I, K) and 3 (J, L) dpf. Labeling is detected in wild type habenular commissure axons, with stronger label in the commissure at 3 dpf (I, arrow). Mutant axons are labeled at 2 dpf (K, arrow), but not at 3 dpf (L). rHb: right habenula; lHb: left habenula. |
|
wnt4a and dnRyk affect commissure formation. Lateral (A) and dorsal (B) view of a 48 hpf embryo, showing expression of wnt4a in the medial habenula. (C) A 3 dpf embryo after injection with a morpholino (MO1) to wnt4a. Habenular afferents have entered the habenula and reached the medial edge (arrows) but have not crossed the roof plate. Axons have innervated the habenula neuropil (arrowhead), which normally occurs only after the commissure has formed. (D) An embryo at 3 dpf after unilateral electroporation with HuC:Gal4/UAS:dnRyk-EGFP at 1 dpf. Abnormal loops and branches are seen within the commissure (n = 15). (E, F) Time series of individual axons show that growth cones reach the contralateral boundary, then bifurcate (arrowheads), turn around and recross the midline (see Supplementary Movies 5 and 6). Time series in (E) was taken without a heated stage, causing slower growth. Time is h:min. PHENOTYPE:
|
|
Manipulation of EphB. (A, B) Incubation of a brain in unclustered Ephrin-B2/Fc did not prevent habenular commissure formation (arrowhead). The ligand did bind to commissural axons, as indicated by Alexa 546 anti-Fc antibody (A). (B) Acetylated tubulin labeling, showing axon tracts. (C) An axon expressing truncated EphB3, fused to GFP (arrow). The axon is traversing the roof plate. |

Unillustrated author statements PHENOTYPE:
|
Reprinted from Molecular and cellular neurosciences, 37(2), Hendricks, M., Mathuru, A.S., Wang, H., Silander, O., Kee, M.Z., and Jesuthasan, S., Disruption of Esrom and Ryk identifies the roof plate boundary as an intermediate target for commissure formation, 271-283, Copyright (2008) with permission from Elsevier. Full text @ Mol. Cell Neurosci.