- Title
-
Epigenetic regulators Rbbp4 and Hdac1 are overexpressed in a zebrafish model of RB1 embryonal brain tumor, and are required for neural progenitor survival and proliferation
- Authors
- Schultz, L.E., Haltom, J.A., Almeida, M.P., Wierson, W.A., Solin, S.L., Weiss, T.J., Helmer, J.A., Sandquist, E.J., Shive, H.R., McGrail, M.
- Source
- Full text @ Dis. Model. Mech.
|
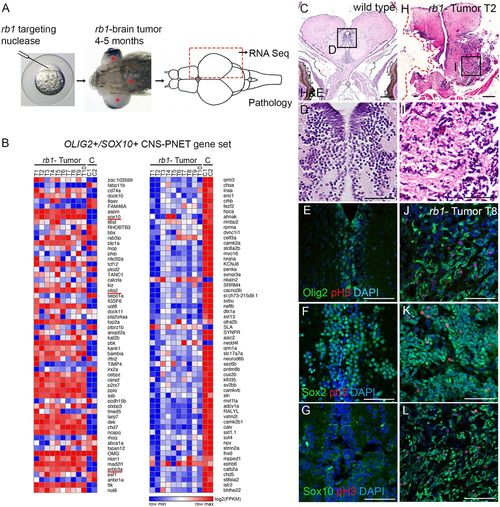
Zebrafish rb1 brain tumors express an oligoneural precursor signature similar to human OLIG2+/SOX10+ CNS-PNET. (A) Zebrafish rb1 brain tumor model created by somatic targeting of rb1 exon 2 with CRISPR-Cas9 or TALENs. Gross brain tumors develop in the midbrain and hindbrain (red asterisks) of adults at 4-5 months of age. For transcriptome analysis, half of the tumor tissue was dissected from ten tumor-positive fish for RNA isolation. The remaining tissue was embedded for pathology and immunolabeling. (B) Heat maps of log2(FPKM) show differential gene expression in zebrafish rb1 brain tumor transcriptome of 120 genes in the human OLIG2+/SOX10+ CNS-PNET subtype. rb1 tumor RNA-Seq of ten biological replicates from ten individual tumor positive adults, and C RNA-Seq of two biological replicates of three pooled normal adult zebrafish brain, are shown. (C-L) Histological and immunolabeling of wild-type adult zebrafish pretectum/diencephalon (C-G) and tumor-containing brain tissue from transcriptome individual T2 (H,I) and T8 (J-L). Neoplastic cells in T2 tumor show small densely basophilic nuclei, consistent with primitive neuroectodermal-like tumors (H,I). In normal adult brain, Olig2, Sox2 and Sox10 labeling is restricted to cells at or adjacent to the ventricle (E-G). High levels of Olig2, Sox2 and Sox10 are detected in the neoplastic tissue in tumor T8. Phosphohistone H3-labeled mitotic cells are scattered throughout the lesion (J-L). Scale bars: 200 µm (C,H); 50 µm (E,F,G,J,K,L). |
|
Zebrafish rb1Δ7/Δ7 homozygous mutant larval brain transcriptome and neurogenic phenotype. (A) Zebrafish rb1/rb1 mutant transcriptome was generated from rb1Δ7/Δ7 homozygous and +/+ wild-type siblings from a cross between heterozygous rb1Δ7/+ adults. Heads from 5 dpf larva were dissected and trunk tissue genotyped. Three pools of five heads of each genotype were used to generate RNA-Seq libraries. (B,C) Wild-type (B) and rb1Δ7/Δ7 (C) 5 dpf larvae. (D) Diagram of larval midbrain and retina with location of neural stem and progenitor cells at brain ventricle (V) in the optic tectum (OT) and thalamic region (Th), and at the retina ciliary marginal zone (cmz). Mature, postmitotic neurons are located in the lateral brain parenchyma and inner retina. (E-L) Immunolocalization with neural differentiation marker HuC/D and mitotic M-phase marker phosphohsitone H3 (E,F,I,J). Wild-type brain and retina show the organization of mature neurons in dorsal brain optic tectum (OT), ventral brain thalamus (Th), and laminated layers of the retina (gcl, ganglion cell layer; inl, inner nuclear layer; onl, outer nuclear layer). Only one mitotic cell is detected at the brain ventricle (F, arrow). Mutant brain shows M-phase cells scattered throughout the midbrain tectum and thalamus (G,H). In the retina, numerous M-phase cells are present across the entire inner nuclear layer, with occasional cells in the outer nuclear and ganglion cell layer (K,L). (M,N) Quantification of pH3-positive cells in midbrain (M) and retina (N) sections from +/+ and rb1Δ7/Δ7 individual 5 dpf larvae (n=16 for each genotype). Data are mean±s.e.m. P-values calculated by two-tailed unpaired Student's t-test. Scale bars: 200 µm (B,C); 50 µm (E,I,G,K); 20 µm (F,J,H,L). |
|
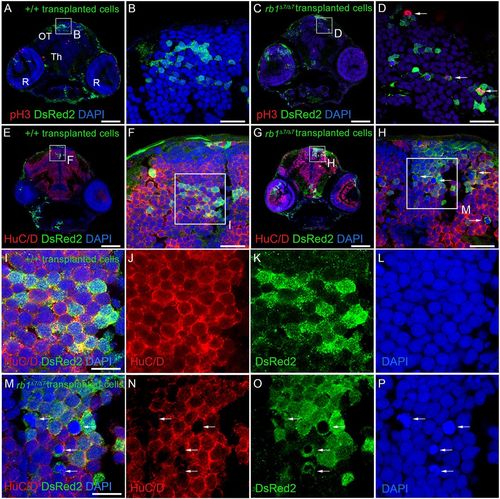
Cell-autonomous requirement for RB1 in blocking cell cycle entry in zebrafish neural precursors. Immunolabeling of 5 dpf host larval brain sections containing descendants of +/+; ubi:DsRed2 and rb1Δ7/Δ7; ubi:DsRed2 donor transplanted cells. n=3 individual transplant experiments for each donor genotype. (A-D) Phosphohistone H3- and DsRed2-labeled sections through the midbrain optic tectum (OT), thalamus (Th) and retinas (R). Wild-type (A,B) and rb1Δ7/Δ7 mutant (C,D) cells present in a wild-type host optic tectum (OT). A few phosphohistone H3-positive rb1Δ7/Δ7 mutant cells can be detected (D, arrows). (E-H) Neuronal marker HuC/D- and DsRed2-labeled sections. Wild-type (E,F) and rb1Δ7/Δ7 mutant (G,H) cells in a host optic tectum express HuC/D. A number of rb1Δ7/Δ7 mutant cells with highly condensed chromatin lack HuC/D labeling (H, arrows). (I-J) Boxed region in F, showing HuC/D- and DsRed2-positive wild-type cells with interphase chromatin. (M-P) Boxed region in H, showing that DsRed2-positive rb1Δ7/Δ7 mutant cells with interphase chromatin also express HuC/D. HuC/D is absent from rb1Δ7/Δ7 mutant cells with highly condensed chromatin (arrows). Scale bars: 100 µm (A,C,E,G); 20 µm (B,D,F,H); 10 µm (I,M). |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
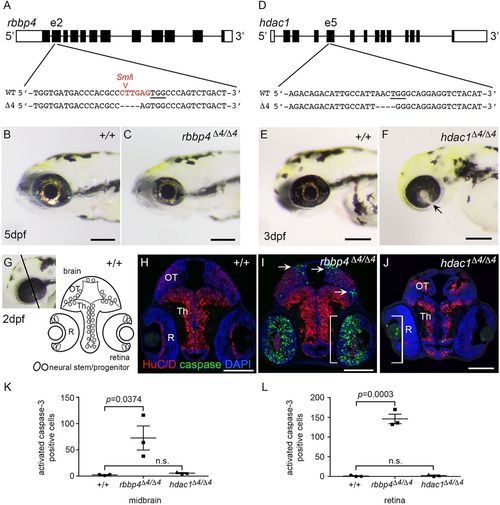
Requirement for chromatin remodelers rbbp4 and hdac1 in zebrafish neurogenesis. (A) CRISPR target site in exon 2 of rbbp4 used to isolate 4 bp frameshift mutation rbbp4Δ4-is60. PAM sequence is underlined. SmlI restriction enzyme site overlapping the target site is shown in red. (B) 5 dpf wild-type (+/+) larva. (C) Gross phenotype of 5 dpf homozygous mutant rbbp4Δ4/Δ4 larva showing microcephaly and microphthalmia. (D) CRISPR target site in exon 5 of hdac1 used to isolate 4 bp frameshift mutation hdac1Δ4-is70. PAM sequence is underlined. (E) 3 dpf wild-type larva. (F) Gross phenotype of 3 dpf homozygous mutant hdac1Δ4/Δ4 larva showing microcephaly and retinal coloboma (arrow). (G) Diagram of 2 dpf larval midbrain and retina. (H-J) Sections of wild-type (H), rbbp4Δ4/Δ4 (I) and hdac1Δ4/Δ4 (J) 2 dpf larval heads labeled with neural differentiation marker HuC/D (red) and apoptosis marker activated caspase 3 (green). In rbbp4Δ4/Δ4, apoptosis is present in the dorsal and lateral tectum (arrows), and throughout the inner retina (brackets) (I). hdac1Δ4/Δ4 larval brain and retina are smaller than wild type, but few apoptotic cells are detected in the brain or retina (J). (K,L) Quantification of activated caspase 3 in the midbrain (K) and retina (L) of 2 dpf wild-type, rbbp4Δ4/Δ4 and hdac1Δ4/Δ4 larvae. OT, optic tectum; Th, thalamus; R, retina. Scale bars: 500 µm (B,C); 200 µm (E,F); 100 µm (H-J). |
|
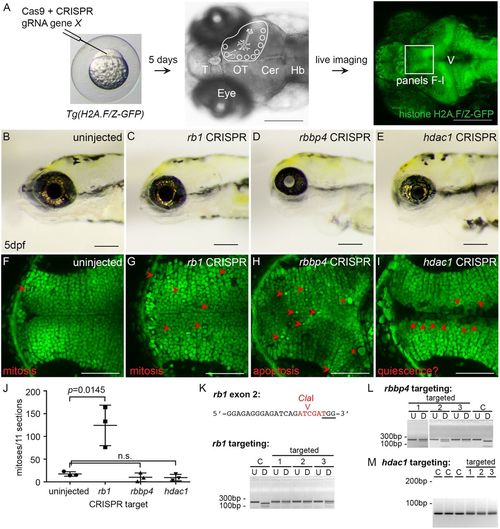
CRISPR somatic targeting-live imaging assay to test chromatin remodeler function in neural cell populations during brain development. Somatic inactivation recapitulates germline mutants and reveals distinct requirements for rb1, rbbp4 and hdac1 in neural progenitor proliferation, differentiation and survival. (A) CRISPR targeting in histone H2A.F/Z-GFP fusion line and dorsal view, and confocal live imaging of GFP in the brain at 5 dpf. (B) 5 dpf wild-type larvae. (C-E) Gross defects in 5 dpf rb1 (C), rbbp4 (D) and hdac1 (E); CRISPR targeted larvae recapitulate germline phenotypes. (F) Confocal imaging in the optic tectum in the brain of a live 5 dpf larva. Section through the ventral region of the optic tectum. Mitotic figures are rarely captured in uninjected larvae at this level of the tectum (red arrowhead). (G) Tectum in rb1 CRISPR-targeted larva shows cells with nuclei containing condensed chromatin (red arrowheads) scattered throughout the postmitotic region of the tissue. (H) Tectum in rbbp4 CRISPR-targeted larva shows intense, punctate fluorescence in presumed apoptotic nuclei (red arrowheads) and abnormally large, irregular nuclei (asterisks). (I) Tectum in hdac1 CRISPR targeted larva shows an overall reduction in size and cell number. Enlarged nuclei with faint GFP line in the ventricle (red arrowheads) are observed. (J) Quantification of M-phase phosphohistoneH3-positive nuclei in the optic tectum in three uninjected control, rb1-, rbbp4- and hdac1-targeted larvae. Targeting experiments were replicated three times for rb1, and two times for rbbp4 and hdac1. Plot shows mean±s.e.m. number of positive nuclei in 11 sections, in three biological replicates. The number of M-phase nuclei in rb1-targeted larvae is significantly different from that in control larvae. rb1, P=0.0145; rbbp4, P=0.3024; hdac1, P=0.1841; n.s., nonsignificant (two-tailed unpaired Student's t-test). (K-M) PCR genotyping shows highly efficient mutagenesis in the three CRISPR-targeted individuals used for quantification. C, control uninjected individuals. Location of rb1 exon 2 CRISPR gRNA and ClaI enzyme site overlapping Cas9 cut site (K). U, undigested PCR amplicon; D, ClaI-digested PCR amplicon surrounding exon 2. rbbp4 and hdac1 targeting was performed with the gRNAs used to isolate loss of function alleles described in Fig. 5. rbbp4 targeting (L). U, undigested exon 2 PCR amplicon; D, SmlI-digested exon 2 PCR amplicon. In the hdac1-targeted larvae the diffuse PCR amplicon band on the gel in targeted individuals correlates with efficient mutagenesis (M). Scale bars: 200 µm (A-E); 50 µm (F-I). |
|
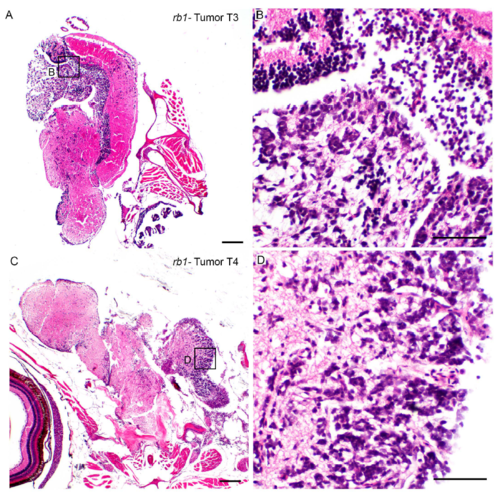
Histological analysis of neoplastic populations in TALEN induced rb1-brain tumor T3 and T4 is consistent with primitive neuroectodermal-like tumor. H&E stained sections of tissue remaining after dissection of tumors T3 (A, B) and T4 (C, D) used for transcriptome analysis. Sections exhibit an unencapsulated, multifocally infiltrative neoplastic population within the neuropil. Neoplastic cells are small with deeply basophilic, round to oval to wedge-shaped nuclei, densely clumped chromatin, and scant amounts of cytoplasm. Scale bars A, C 200μm; B, D 50 μm. |
|
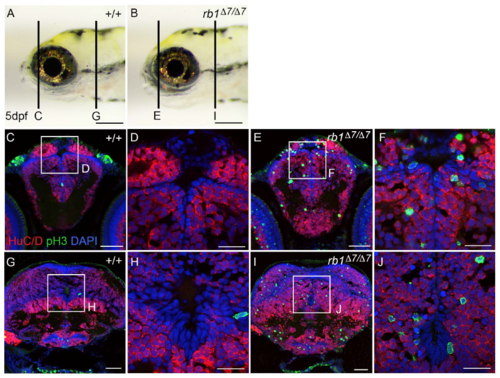
Ectopic M-phase phosphohistone-H3 positive cells in the telencephalon/forebrain and hindbrain of rb1D7/D7 mutant 5 dpf larva. A wildtype +/+ and B rb1D7/D7 mutant 5 dpf gross morphology. C-J Cryosections were labeled with antibodies to the neuronal marker HuC/D (red) and mitotic M-phase marker phosphohistone-H3 (green). Sections through the telencephalon (C-F) and hindbrain (G-J) show that in wild type mitotic cells are absent or rare, but in the rb1D7/D7 mutant many ectopic phosphohistone-H3 positive cells are scattered throughout the region containing HuC/D-positive cell bodies of neurons. |
|
rb1D7/D7 mutant cells in M-phase lack expression of the neuronal differentiation marker HuC/D. A, B High magnification image of phosphohistone-H3 positive nuclei (B, arrows) and HuC/D-positive cells in the tectum of a 5 dpf rb1D7/D7 mutant brain. No overlap is detected between cells expressing HuC/D (C, red) and phosphohistone-H3 (D, green, arrows, dashed outlines). The chromatin in the phosphohistone-H3 positive cells appears highly condensed (E, arrows), consistent with cells in prophase/prometaphase of the cell cycle. |