- Title
-
Activation of the hypothalamic feeding centre upon visual prey detection
- Authors
- Muto, A., Lal, P., Ailani, D., Abe, G., Itoh, M., Kawakami, K.
- Source
- Full text @ Nat. Commun.
|
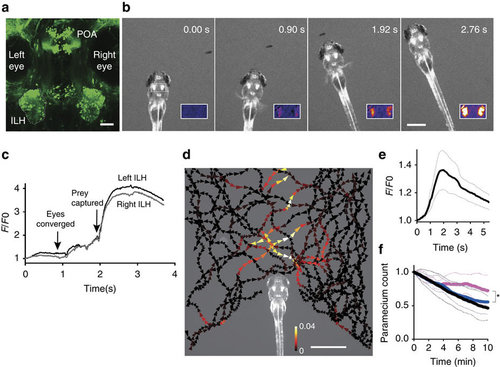
Activity in the ILH in the presence of prey. (a) UAS:EGFP reporter gene expression in the ILH in hspGFFDMC76A Gal4 fish at 5?d.p.f. The left ILH is encircled by dotted lines. ILH: the inferior lobe of the hypothalamus, POA: preoptic area. Scale bar, 50??m. (b) ILH activation during prey capture behaviour in a previously unfed 4?d.p.f. larva. Selected frames from a time-lapse fluorescent microscopy movie of UAShspzGCaMP6s; hspGFFDMC76A larvae. Inset: F/F0 in pseudo-colours. Scale bar: 500??m. (c) Normalized GCaMP6s fluorescence intensity (F/F0) from the recording shown in b. (d) ILH activity in the presence of a paramecium in a 5?d.p.f. zebrafish larva that was embedded in agarose. The trajectories of a single paramecium over 149?s are shown with the colour-coded changes in the intensity of the GCaMP6s fluorescence in the ILH. The signals in the left and right ILH were averaged. The length of the arrowhead indicates the distance travelled by the paramecium in 120?ms. Scale bar, 1?mm. (e) ILH activity (GCaMP6s fluorescence intensity, F/F0) in response to a moving spot on a screen. (Mean (thick line)ąs.e.m. (thin dotted lines), n=5 larvae). The signals measured in the left and right ILH were averaged. A moving spot of 2.4° in diameter was presented to the larva at a speed of 100?deg?s?1 from 0 to 3.1?s. (f) Paramecium consumption in UAS:zBoTxBLCGFP; hspGFFDMC76A larvae (n=6, magenta), control larvae (UAS:zBoTxBLCGFP alone) (n=18, blue), and wild-type control larvae (n=13, black). The number of the paramecia left in the chamber was normalized to the initial count. The thick lines represent the mean and the thin dotted lines represent the s.d. The asterisk indicates a significant difference between the toxin:Gal4 double transgenic larvae and UAS:zBoTxBLCGFP alone control larvae at 10?min (one-tailed t-test, P=0.040). |
|
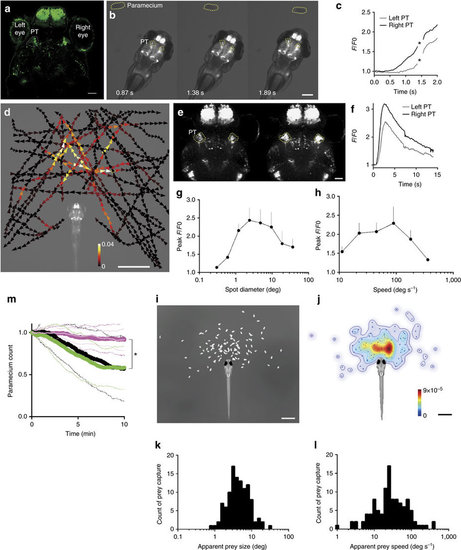
Activity in a subset of cells in the pretectal area in the presence of prey. (a) UAS:EGFP reporter gene expression in the pretectal area (PT, encircled in dotted lines) in gSAzGFFM119B Gal4 larva at 5?d.p.f. Scale bar, 50??m. (b) Pretectal activation in response to prey at 4?d.p.f. GCaMP6s fluorescence images. Scale bar, 500??m. (c) GCaMP6s fluorescence intensity in the pretectal areas shown in b. Asterisks: missed data points due to large movements of the larva. (d) Averaged activity of the bilateral pretectal areas in the presence of prey in a 6?d.p.f. larva embedded in agarose. The trajectories of a single paramecium over 107?s with colour-coded changes in the intensity of GCaMP6s fluorescence in the pretectal areas. Scale bar, 1?mm. (e) Confocal images showing pretectal activation in response to a moving spot. Scale bar, 50??m. (f) GCaMP6s fluorescence intensity obtained from the time-lapse images in e. A moving spot of 2.4° in diameter was presented to a larva at a speed of 100?deg?s?1 from 0.3 to 3.4?s. (g,h) Size-tuning and speed?tuning curve, respectively (n=6 larvae at 4?d.p.f.; Meanąs.e.m.). Maximal responses to a moving spot. The x-axis is logarithmically scaled. (i) Relative positions of the paramecium at the onset of prey capture in free-swimming larvae at 5?d.p.f. (n=110 successfully completed prey capture events by 6 larvae). Scale bar, 1?mm. j, Probability density of the paramecium positions shown in i. Colour bar:0 (blue) and 9 × 10?5 (red). Scale bar, 1?mm. (k,l) Histograms of the apparent sizes and speeds, respectively, of the prey at the onset of prey capture from the data shown in i. (m) Paramecium consumption in the UAS:EGFP; gSAzGFFM119B larvae that were subjected to bilateral pretectal ablation (n=10, thick magenta line) or unilateral (n=10, thin light-magenta line), UAS:EGFP; gSAzGFFM119B larvae subjected to bilateral olfactory bulb ablation (n=11, green) and UAS:EGFP; gSAzGFFM119B larvae (no laser irradiation, n=8, black). Solid lines:mean. Dotted lines: s.d. Asterisks: significant differences between each of pretectum ablation groups and each of control groups at 10?min (Tukey?s HSD test, P=0.0016 for bilateral pretectum ablation and olfactory bulb ablation). |
|
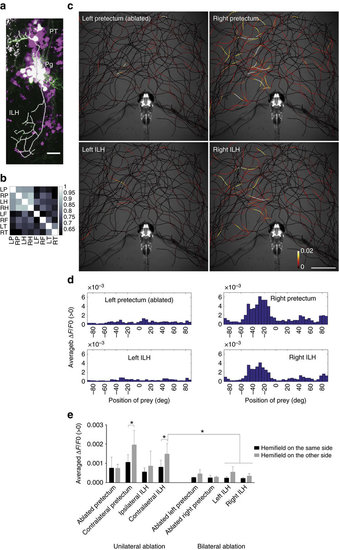
Anatomical and functional connections from the pretectum to the ILH. (a) Merged image of the pretectal cells that were sparsely labelled by the injection of UAS:EGFP DNA (EGFP in green) in the UAS:RFP; gSAzGFFM119B background (red fluorescent protein shown in magenta). The axonal projection of a single pretectal cell is shown as a thick white line. Scale bar, 20??m. (b) Correlation of the activity of the pretectum and ILH. UAShspzGCaMP6s; hspGFFDMC76A; gSAzGFFM119B double Gal4 transgenic larvae were imaged and the cross-correlation coefficients of the calcium signals in the specified areas were calculated (Colour-coded, 1: highest correlation, 0: no correlation). (c) Activities in the pretectum and ILH in the presence of a paramecium in a 5?d.p.f. larva that was embedded in agarose. The trajectories of a single paramecium over 318?s are shown with colour-coded changes in the intensity of GCaMP6s fluorescence in a 5?d.p.f. UAShspzGCaMP6s; hspGFFDMC76A; gSAzGFFM119B double Gal4-transgenic larva that was subjected to laser ablation of the left pretectum. Scale bar, 1?mm. (d) Preference for the azimuthal position of the paramecium. (e) Left-right hemifield preference in the pretectum and ILH in 5?d.p.f. larvae that were subjected to unilateral pretectum ablation (bar graphs on the left) and abolition of the neuronal activity by bilateral pretectum ablation (bar graphs on the right). Meanąs.d. The asterisks indicate significant differences. ?Contralateral pretectum?: unilaterally ablated larvae n=6, two-tailed t-test, P=0.0261. ?Contralateral ILH?: unilaterally ablated larvae n=6, two-tailed t-test, P=0.0325. ?Contralateral ILH???Left ILH? and ?Right ILH?: bilaterally ablated larvae n=4, Tukey?s HSD test, less than P<0.0034 for all 4 groups. On the x-axis are the brain areas on which ROIs were set to measure the GCaMP6s fluorescence intensities. |
|
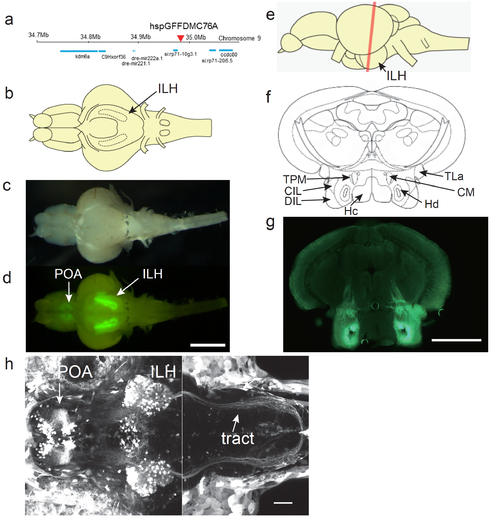
UAS:EGFP reporter gene expression in the hspGFFDMC76A Gal4 line a, Insertion site of the hspGFFDMC76A was identified in an intergenic region on chromosome 9. b. Schematic of a ventral view of the brain of an adult zebrafish. c, Ventral view of a dissected brain of an adult UAS:EGFP;hspGFFDMC76A fish. d, EGFP fluorescence in the brain shown in b. Scale bar: 1 mm. e, Schematic side view of the adult brain. The red line indicates the positions of the sections in f and g. f, Annotations based on Wulliman et al., 1996. g, EGFP fluorescence of a coronal section of the brain of an adult UAS:EGFP;hspGFFDMC76A zebrafish. Scale bar: 0.5 mm. h, UAS:EGFP expression in the hspGFFDMC76A Gal4 line at 5 dpf. Projected z-stack images obtained by two-photon laser microscopy. Left: z-stack projection of 155 slices (1 ?m-step). Right: z-stack projection of 135 slices (1 ?m-step). The positions of the focal planes of the two z-stacks overlap by 35 um (the right one more dorsal) along z-axis. Scale bar: 50 ?m. CIL, central nucleus of the inferior lobe; CM, corpus mamillare; DIL, diffuse nucleus of the inferior lobe; Hc, caudal zone of periventricular hypothalamus; Hd, dorsal zone of periventricular hypothalamus; ILH, inferior lobes of the hypothalamus; POA, preoptic area; TLa, torus lateralis; TPM, tractus pretectomamillaris. |
|
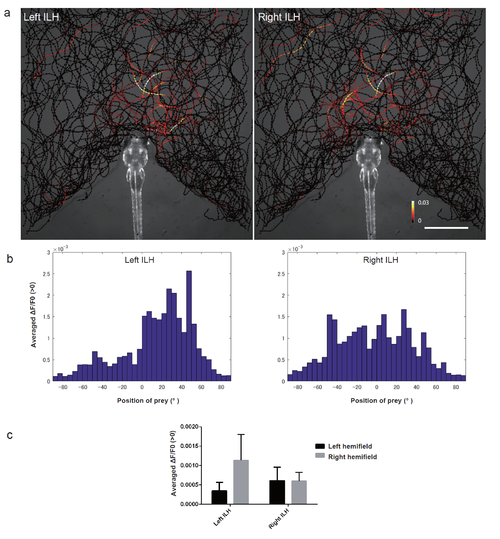
Activity in the ILH at the sight of prey a, The trajectories of single paramecia over 942 s are shown with the colour-coded changes in the intensity of GCaMP6s fluorescence in the left and right ILH areas in 5 dpf UAShspGCaMP6s;hspGFFDMC76A larvae. The data from 4 larvae were merged into a single larval image. Scale bar: 1 mm. The length of the arrowhead indicates the distance travelled by the paramecium in 60 ms. b, Average increase in the Ca signals in each 5° bin. The data are the same as those shown in a. c, Average increase in the Ca signals in the left and right hemifields. The data are the same as those shown in a. Mean ą S.D. |
|
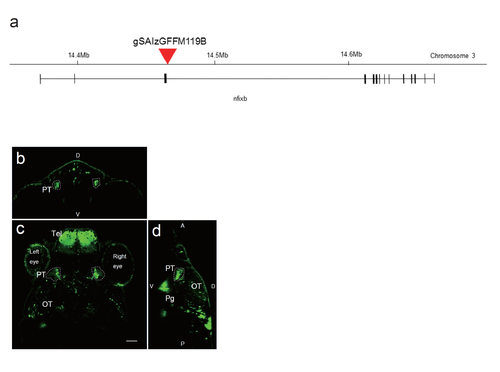
The Gal4 insertion site in the gSAIzGFFM119B gene-trap Gal4 line and UAS:EGFP reporter gene expression a, Insertion site of the Gal4 construct in the gSAIzGFFM119B line. The insertion site was identified within the nfixb gene on chromosome 3. b-d, Confocal images of the expression of the UAG:EGFP reporter, driven by Gal4 in the gSAzGFFM119B line. b, Coronal optical section. c, Dorsal view of a single optical section showing the positions of the pretectal cells. Scale bar: 50 ?m. d, Sagittal view. D, dorsal; V, ventral; PT, pretectum; Tel, telencephalon; OT, optic tectum; Pg, preglomerular nuclei. |
|
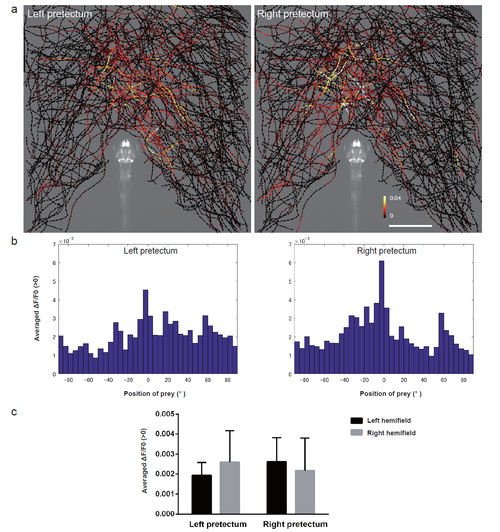
Activity in the pretectal cells at the sight of prey a, The trajectories of single paramecia over 775 s are shown with the colour-coded changes in the intensity of GCaMP6s fluorescence in the pretectum in 5 dpf UAShspGCaMP6s; gSAIzGFFM119B larvae. The data from 4 larvae are merged into a single larval image. Scale bar: 1 mm. The length of the arrowhead represents the distance travelled by the paramecium in 60 ms. b, Average increase in the Ca signals in each 5° bin. The data are the same as those shown in a. c, Averaged increase in the Ca signals in the left and right hemifields. The data are the same as those shown in a. Mean ą S.D. |
|
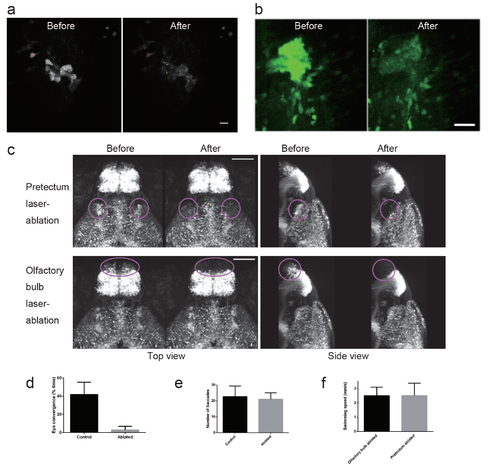
Ablation of the pretectal cells in UAS:EGFP;gSAIzGFFM119B larvae a, Images of the EGFP fluorescence of the pretectal cells on a focal plane before and after two-photon laser ablation in a 4 dpf UAS:EGFP;gSAIzGFFM119B larva. The targeted cells are labelled with small coloured circles. Scale bar: 10 ?m. b, Image of the EGFP fluorescence of the pretectal cells in a UAS:EGFP;gSAIzGFFM119B larva. z-stack images that covered the entire gregion of the 119B-labelled pretectal cells were projected onto one image. Before (left) and after (right) laser ablation. Scale bar: 25 ?m. c, Absence of fluorescent cells after laser ablation. Top panel: pretectum ablation (encircled in magenta). Bottom panel: olfactory bulb ablation (encircled in magenta) as a control experiment. Scale bar: 100 ?m. d, Eye convergence (indicator of prey capture, mean ą S.D.; Control, n = 3 untreated larvae; Ablated, n = 5 pretectum-ablated larvae; two-tailed ttest, p = 0.0008). e, Optokinetic response (mean ą S.D.; Control, n = 8 untreated larvae; Ablated, n = 6 pretectum-ablated larvae; two-tailed t-test p = 0.5920). f, Locomotor activity in olfactory bulb-ablated larvae (n=11) and pretectum-ablated larvae (n=10). The average swimming speeds in 10 min recordings are shown with S.D. |
|
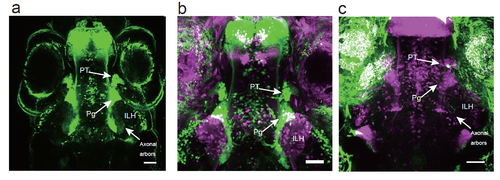
Projection of the 119B-pretectal cells to the ILH a, Merged image of 3 optical sections (66.7 ?m and 22 ?m apart on the Z-axis in the ventral direction) depicting the pretectal area (PT), the preglomerular nuclei (Pg), and the axonal arbours of the pretectal cells that reached the ILH at 5 dpf. Scale bar: 50 ?m. b, Merged image of a UAS:EGFP;gSAIzGFFM119B larva (green) and a UAS:EGFP;hspGFFDMC76A larva (magenta, a different individual), which shows the location of the ILH relative to the PT and Pg. Scale bar: 50 ?m. c, A single pretectal (PT) cell labelled by a UAS:EGFP DNA injection (green) into a UAS:RFP;gSAIzGFFM119B background zebrafish (magenta) at the 1-cell stage and observed at 4 dpf. An axon is extending out of the soma of the labelled cell in the pretectal area and is projecting to the caudomedial ILH. Scale bar: 50 ?m. |
|
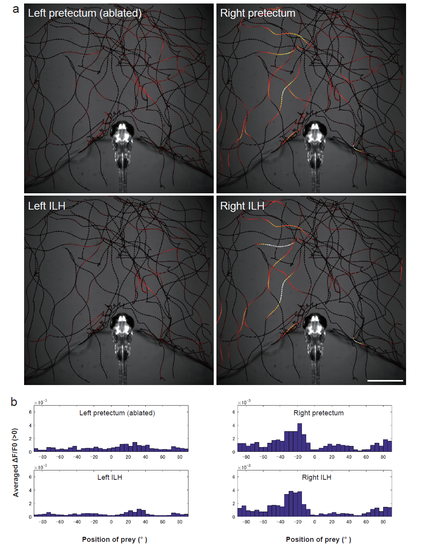
Activity in the pretectum and ILH at the sight of prey in a larvae subjected to unilateral pretectum ablation a, Another example of larvae in the unilateral ablation experiments shown in Figure 3c. The trajectories of a single paramecium over 250 s are shown with the colour-coded changes in the intensity of GCaMP6s fluorescence in a 5 dpf UAShspGCaMP6s; hspGFFDMC76A; gSAIzGFFM119B larva that was subjected to laser ablation of the left pretectum. Scale bar: 1 mm. The length of the arrowhead represents the distance travelled by the paramecium in 60 ms. b, Average increase in the Ca signals in each 5° bin. The data are the same as those shown in a. |
|
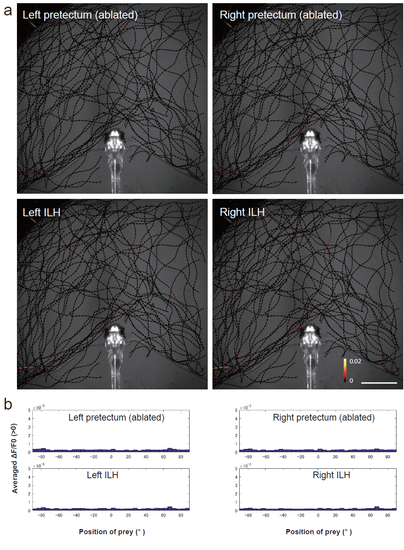
Activity in the pretectum and ILH at the sight of prey in a larvae subjected to bilateral pretecutm ablation a, A representative example of the larvae in the bilateral ablation experiments. The trajectories of single paramecia over 324 s are shown with the colour-coded changes in the intensity of GCaMP6s fluorescence in a 5 dpf UAShspGCaMP6s;hspGFFDMC76A; gSAIzGFFM119B larva that was subjected to laser ablation of the bilateral pretectum. Scale bar: 1 mm. The length of the arrowhead represents the distance travelled by the paramecium in 60 ms. b, Average increase in the Ca signals in each 5° bin. The data are the same as those shown in a. |
|
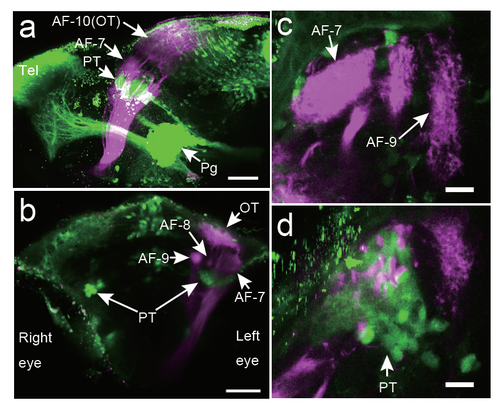
Locations of the 119B-pretectal area and the retinal ganglion cell arbours Locations of the axonal arbourisation fields (AFs) of the ganglion cells (magenta) relative to the gSAzGFFM119B-labelled cells (green). DiI was injected into the right eye, and the left eye was removed for observation. a, Lateral view from the left side. Scale bar: 50 ?m. b, Front view. Scale bar: 50 ?m. c, Top view (focused on the AF-7 area). Scale bar: 5 ?m. d, Top view (focused 26 ?m below the AF-7 area). Scale bar: 5 ?m. PT, pretectal area; OT, optic tectum; Tel, telencephalon; Pg, preglomerular nuclei; D, dorsal; V, ventral; A, anterior; P, posterior. |