- Title
-
Tbx1 controls the morphogenesis of pharyngeal pouch epithelia through mesodermal Wnt11r and Fgf8a
- Authors
- Choe, C.P., Crump, J.G.
- Source
- Full text @ Development
|
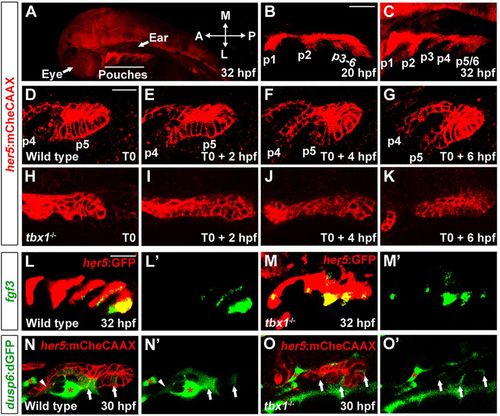
Requirement of Tbx1 for pouch morphogenesis. (A) A zoomed out view of a 32hpf her5:mCherryCAAX (red) embryo shows the position of pouches relative to the developing eye and ear. (B,C) The first two pouches (p1, p2) form by 20hpf, with the remaining pouches (p3-p6) developing at one per 4h. (D-K) Still images from time-lapse confocal recordings of wild-type and tbx1 mutant pouch development at the times indicated (bottom right of the images) (see supplementary material Movie 1A,B). her5:mCherryCAAX labels endodermal cell membranes. Recordings were started at 26hpf (T0), a stage when the fourth and fifth pouches (p4 and p5) are beginning to develop in the wild-type example. Compared with the typical pouch outpocketing behavior seen in all three wild-type siblings, no endodermal outpocketing was observed in three out of three tbx1 mutants (H-K). (L,M) Fluorescent in situ hybridization for fgf3 (green) and GFP immunohistochemistry to label the her5:GFP-positive pouch endoderm (red). fgf3 was expressed in a segmental pattern in all 39 wild-type siblings and all 14 tbx1 mutants. (N,O) Confocal sections of pre-pouch endoderm in dusp6:dGFP; her5:mCherryCAAX transgenic embryos. In wild type, dusp6:dGFP (green) was expressed segmentally in already formed pouches (arrowhead) and in clusters of presumptive pouch endoderm before outpocketing (arrows); strong expression was also seen in adjacent mesoderm (asterisks) (n=18). In tbx1 mutants, dusp6:dGFP was expressed at lower levels and in fewer cells, yet a segmental pattern in the endoderm was still detected (n=7). Note that we increased the relative gain of the green channel in tbx1 mutants to reveal the weak segmental dusp6:dGFP expression. See supplementary material Fig. S1 for the unaltered images. L′-O′ show the green channel alone. Scale bars: 40μm (B,C,L,M); 20μm (D-K,N,O). EXPRESSION / LABELING:
|
|
Tbx1 is required in the nkx2.5-positive mesoderm for pouch development. (A-E) Fluorescent in situ hybridization for tbx1 (green) and GFP immunohistochemistry to detect her5:GFP-positive endoderm (red) at 34hpf. In all 58 wild-type siblings, tbx1 expression was observed in her5:GFP-positive endoderm (arrows), adjacent mesoderm (arrowheads) and the ear (asterisk). In all 21 tbx1 mutants, all tbx1 expression was lost. tbx1 expression was selectively restored in the her5:GFP-positive endoderm of all 17 nkx2.3:Tbx1; tbx1-/- embryos and in the mesoderm of all nine nkx2.5:Tbx1; tbx1-/- embryos. Inclusion of both transgenes restored tbx1 expression over a broader area (brackets for presumptive endoderm and arrowheads for presumptive mesoderm) in all four nkx2.3:Tbx1; nkx2.5:Tbx1; tbx1-/- embryos, although for technical reasons her5:GFP was not included. (F-J) In wild-type zebrafish at 34hpf, immunohistochemistry for Alcama (green) labeled five pouches (p1-p5). nkx2.5:Tbx1; tbx1-/- and nkx2.3:Tbx1; nkx2.5:Tbx1; tbx1-/- embryos displayed partial rescue of pouches compared with tbx1 mutants, whereas nkx2.3:Tbx1; tbx1/ embryos did not. Sensory ganglia are indicated with red asterisks. (K-O) Fluorescent in situ hybridization for dlx2a (green) and GFP immunohistochemistry for her5:GFP (red) at 30hpf. In wild-type zebrafish, dlx2a was expressed in the neural-crest-derived mesenchyme of each arch (numbered), with higher expression in posterior arches. In all tbx1-/- (n=9), nkx2.3:Tbx1; tbx1/ (n=6), nkx2.5:Tbx1; tbx1-/- (n=4) and nkx2.3:Tbx1; nkx2.5:Tbx1; tbx1-/- (n=4) embryos, dlx2a expression was reduced in the second arch and lost from the more posterior arches. For technical reasons, her5:GFP was not included in O. (P-T) Ventral whole-mount views of dissected facial cartilages. Wild-type zebrafish (P) invariantly formed five ceratobranchials (CBs) on each side. CBs were missing in all tbx1 mutants (Q, n=41) and not recovered in nkx2.3:Tbx1; tbx1-/- (R, n=23), nkx2.5:Tbx1; tbx1/ (S, n=26) or nkx2.3:Tbx1; nkx2.5:Tbx1; tbx1/ (T, n=24) embryos. (U) Quantification of pouch defects as assessed by Alcama staining in wild-type zebrafish (n=342), tbx1-/- (n=63), nkx2.3:Tbx1; tbx1-/- (n=54), nkx2.5:Tbx1; tbx1-/- (n=22) and nkx2.3:Tbx1; nkx2.5:Tbx1; tbx1-/- (n=23). Data represent mean±s.e.m., P values are shown. n.s., not significant. Scale bars: 40μm (A-O). |
|
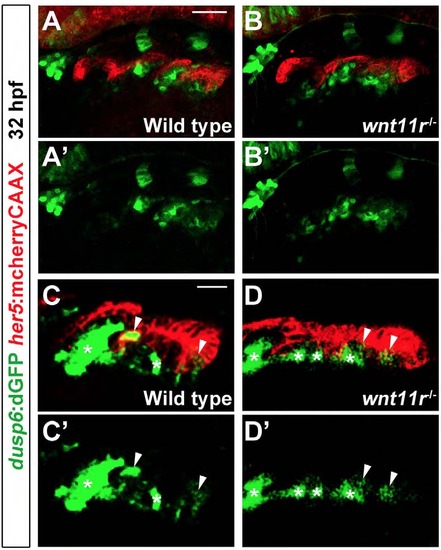
Rescue of tbx1-/-pouch defects with mesodermal Fgf8a and Wnt11r. (A-J) Fluorescent in situ hybridization for fgf8a or wnt11r (green) and GFP immunohistochemistry to detect nkx2.5:GFP-positive mesoderm (red) at 30hpf. In wild type, fgf8a was expressed in ventral nkx2.5:GFP-positive mesodermal cores of arches 3 and 4 (m3 and m4), whereas wnt11r was in more dorsal subsets of these mesodermal cores. In tbx1 mutants, mesodermal expression of fgf8a (n=12 of 12) and wnt11r (n=10 of 11) was lost, although fgf8a and wnt11r expression in the anterior (arrow) and posterior (arrowheads) portions of the otic vesicle was unaffected. An nkx2.3:Tbx1 transgene did not restore mesodermal fgf8a (n=0 of 7) and wnt11r expression (n=0 of 5), whereas an nkx2.5:Tbx1 transgene restored mesodermal fgf8a (n=5 of 6) and wnt11r expression (n=9 of 9). Also, nkx2.5:Fgf8-GFP and nkx2.5:Wnt11r transgenes restored fgf8a (n=27 of 27) and wnt11r (n=21 of 21) expression, respectively. (K-O) Alcama immunohistochemistry (green) showed five pouches (p1-p5) in wild-type fish at 34hpf. tbx1-/- mutants lost all pouches except for the first (p1), whereas individual nkx2.5:Wnt11r or nkx2.5:Fgf8a-GFP transgenes modestly rescued, and combined nkx2.5:Wnt11r and nkx2.5:Fgf8a-GFP transgenes strongly rescued posterior pouches (p2-p5). Sensory ganglia are indicated with red asterisks. (P-T) Ventral views of dissected facial cartilages. A bilateral set of five CBs formed in wild-type zebrafish, and no CBs formed in tbx1 mutants. No rescue of CB cartilage was seen in nkx2.5:Wnt11r; tbx1-/- (n=39), nkx2.5:Fgf8a-GFP; tbx1-/- (n=34), or nkx2.5:Wnt11r; nkx2.5:Fgf8a-GFP; tbx1-/- larvae (n=31). (U) Quantification of pouch defects based on Alcama staining in wild type (n=51), nkx2.5:Wnt11r (n=49), nkx2.5:Fgf8a-GFP (n=36), tbx1-/- (n=62), nkx2.5:Wnt11r; tbx1-/- (n=44), nkx2.5:Fgf8a-GFP; tbx1-/- (n=37), and nkx2.5:Wnt11r; nkx2.5:Fgf8a-GFP; tbx1-/- (n=24). Data represent mean±s.e.m., P values are shown. (V) Low magnification view of an embryo at 32hpf showing nkx2.5:GFP-positive mesoderm (green) relative to her5:mCherryCAAX-positive pouch endoderm (red) and the developing eye and ear. The schematic shows expression of fgf8a (green) and wnt11r (yellow) within distinct subsets of nkx2.5-positive mesoderm (red) during the formation of endodermal pouches (blue). Scale bars: 40μm (A-O). |
|
Synergistic pouch and cartilage defects in compound wnt11r; fgf8a mutants. (A-D) Alcama immunohistochemistry (green) labeled five pouches (p1-p5) in wild-type embryos at 34hpf. Compared with the mild reductions of pouches in wnt11r or fgf8a single mutants, compound wnt11r-/-; fgf8a-/- mutants had a near complete loss of pouches. Sensory ganglia are indicated with red asterisks. (E-H) Dissections of facial cartilage at 5dpf. Wild type had five CBs on each side, wnt11r and fgf8a single mutants had variable fusions and losses of on average one CB and compound wnt11r-/-; fgf8a-/- mutants lost multiple CBs. (I) Quantification of defects. Number of embryos examined (pouches, CBs): wild type (106, 102), wnt11r-/- (66, 57), fgf8a-/- (68, 98), and wnt11r-/-; fgf8a-/- (24, 20). Data represent mean±s.e.m. ***P<0.001 relative to fgf8a mutants alone. Scale bar: 40μm (A-D). |
|
Requirement for Fgf8a in the directional persistence of pouch cells. (A-J) Representative confocal sections from time-lapse recordings show the development of pouches p4-p6 in a wild type (n=5) and fgf8a mutant (n=3) (see supplementary material Movie 3A,B). her5:mCherryCAAX labels endodermal cell membranes (red) and dusp6:dGFP shows dynamic Fgf activity (green). T0 is 26 hpf. Merged images are shown in A-J and dusp6:dGFP alone in A′′-J′′. Schematics in A′-J′ show the graded intensity of dusp6:dGFP (green) relative to endodermal cells (red). Individual cell tracks used for the quantification are shown in A′′′-J′′′. (K-M) The velocity, persistence of migration and distribution of angles of tracked cells over a 7-h period. For distribution of angles, each bin represents the number of cells moving in a particular direction relative to the last cell position. We tracked the cells of two embryos for each wild type and fgf8a mutant. Mean±s.e.m. and P values are shown. n.s., not significant. Scale bar: 20μm (A-J). |
|
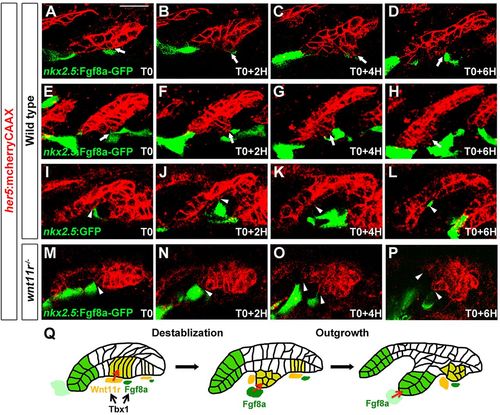
Ectopic Fgf8a redirects pouch outgrowth. (A-P) Still images from time-lapse recordings monitoring the development of the third pouch in wild-type embryos with mosaic mesodermal mis-expression of a nkx2.5:Fgf8a-GFP transgene (two examples are shown: A-D and E-H) or a control nkx2.5:GFP transgene (I-L), as well as wnt11r mutants with nkx2.5:Fgf8a-GFP mis-expression (M-P) (see supplementary material Movie 4). her5:mCherryCAAX labels endodermal cell membranes in red. Recordings started at 25hpf (T0), and subsequent stills were taken every 2h. nkx2.5:Fgf8a-GFP-expressing clones transiently diverted developing third pouch cells (arrows) in four out of six embryos, whereas nkx2.5:GFP-expressing clones had no effect on adjacent third pouch cells (arrowheads) in all three of the embryos examined. nkx2.5:Fgf8a-GFP-expressing clones failed to attract wnt11r-/- endodermal cells (arrowheads) in all embryos (n=3). (Q) A two-step model of Tbx1 function in pouch morphogenesis. Tbx1 promotes wnt11r and fgf8a expression in distinct domains of the mesoderm, with Wnt11r initiating pouch morphogenesis through epithelial destabilization and Fgf8a guiding subsequent pouch outgrowth. Scale bar: 20μm (A-P). |
|
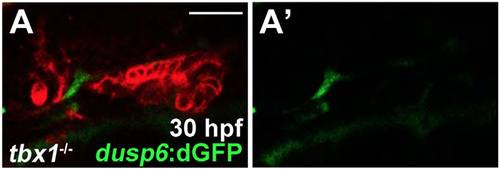
Original image of dusp6:dGFP; her5:mCherryCAAX; tbx1-/- embryos |
|
Mesodermal tbx1 expression during pouch formation and a requirement for Tbx1 in ectomesenchyme development |
|
wnt4a and fzd8a expression in tbx1 mutants and analysis of nkx2.5:Wnt11r and nkx2.5:Fgf8a-GFP transgenes |
|
Interactions between Wnt4a, Wnt11r, and Fgf8a during pouch formation |
|
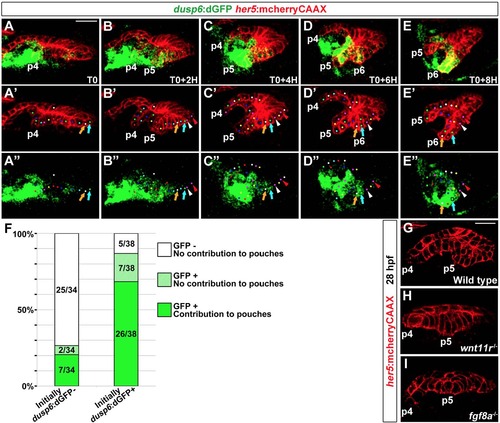
Preferential contribution of dusp6:dGFP+ cells to pouches |
|
Fgf activity in the endoderm of wnt11r mutants |
|
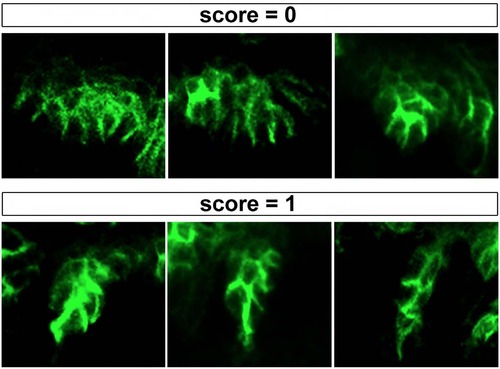
Criteria for scoring pouch rescue |