Figure 2
- ID
- ZDB-FIG-250320-14
- Publication
- Manickam et al., 2025 - Paving the way for better ototoxicity assessments in cisplatin therapy using more reliable animal models
- Other Figures
- All Figure Page
- Back to All Figure Page
|
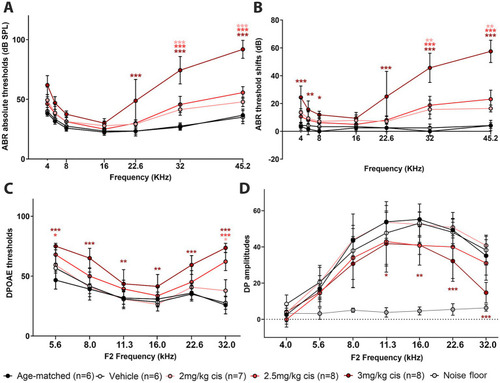
Clinical cisplatin affects hearing function in mice in a dose-dependent manner. Eight weeks old mice (>20 grams) received clinical cisplatin in a multi-cycle protocol. Hearing function was measured before and after the completion of cisplatin treatment. The following groups were tested: age-matched controls (black circles), vehicle (saline solution, white circles), clinical cisplatin at 2 mg/kg b.w. (pink circles), 2.5 mg/kg b.w (red circles) and 3 mg/kg b.w. (marron circles). |