|
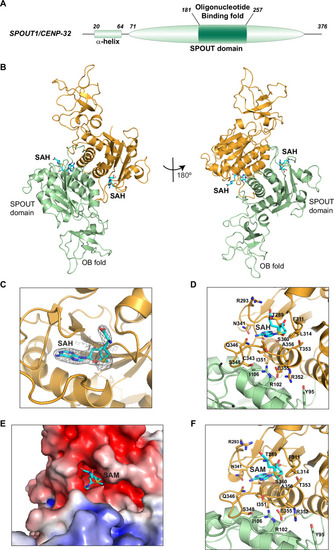
Structural characterization reveals that SPOUT1/CENP-32 is a SPOUT Methyltransferase that dimerizes through its catalytic domain. A Domain architecture of SPOUT1/CENP-32 with the structural domains of SPOUT1/CENP-32 highlighted. B Cartoon representation of the crystal structure of SPOUT1/CENP-32 71-376 bound to S-adenosyl homocysteine (SAH) in two orientations (left and right panel). The main domains of SPOUT1/CENP-32 (SPOUT domain and OB-fold) are highlighted. SAH is bound to the cofactor pocket, indicating that SPOUT1/CENP-32 could be an active methyltransferase. Our structural analysis is consistent with the structure that was deposited by SGC while this manuscript was in preparation (PDB: 4RG1). C, D and F Close-up of the active site of SPOUT1/CENP-32 with SAH (C and D) and with SAM (F) showing the electron density map for SAH (C) and the amino acid residues that are responsible for the interactions that stabilize cofactor binding. The binding of SAH and SAM is similar. E Close-up of the electrostatic surface potential of SPOUT1/CENP-32 71-376 bound to SAM showing the deep pocket formed by the trefoil knot.
|