Figure 5
- ID
- ZDB-FIG-210719-5
- Publication
- Adams et al., 2021 - A two-site flexible clamp mechanism for RET-GDNF-GFRα1 assembly reveals both conformational adaptation and strict geometric spacing
- Other Figures
- All Figure Page
- Back to All Figure Page
|
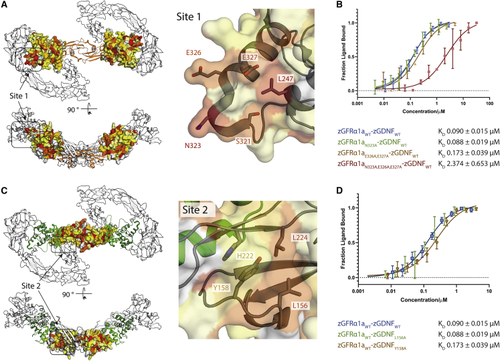
Mutational analysis of zGDNF and zGFRα1 site 1 and 2 interactions with zRETECD (A) Heatmap of the sequence conservation between hGFRα paralogs, and zGFRα1a mapped onto the structure of zGFRα1a D2-D3 domains reported here. Residues are colored by similarity (red highly similar to yellow through to white, least similar). Two orthogonal views are shown. Right panel, close-up of site 1 and conserved zGFRα1a residues. (B) Binding curves and KD values obtained using MST for zGFRα1aD1-3 and mutations assessed in complex with zGDNFmat., with a minimum of n = 3 repeats for the WT and the mutations with the SE represented. (C) Heatmap of the sequence similarity between GDNF paralogs depicted as a surface representation, mapped onto zGDNF138-235. Right panel, close-up of site 2 contact between RETCRD and zGDNF dimer. (D) MST binding curves and KD values for zGDNF and mutations L156A and Y158A probed in complex with WT zGFRα1a binding to zRETECD. |
Reprinted from Structure (London, England : 1993), 29(7), Adams, S.E., Purkiss, A.G., Knowles, P.P., Nans, A., Briggs, D.C., Borg, A., Earl, C.P., Goodman, K.M., Nawrotek, A., Borg, A.J., McIntosh, P.B., Houghton, F.M., Kjær, S., McDonald, N.Q., A two-site flexible clamp mechanism for RET-GDNF-GFRα1 assembly reveals both conformational adaptation and strict geometric spacing, 694-708.e7, Copyright (2021) with permission from Elsevier. Full text @ Structure