Figure 6
- ID
- ZDB-FIG-210719-6
- Publication
- Adams et al., 2021 - A two-site flexible clamp mechanism for RET-GDNF-GFRα1 assembly reveals both conformational adaptation and strict geometric spacing
- Other Figures
- All Figure Page
- Back to All Figure Page
|
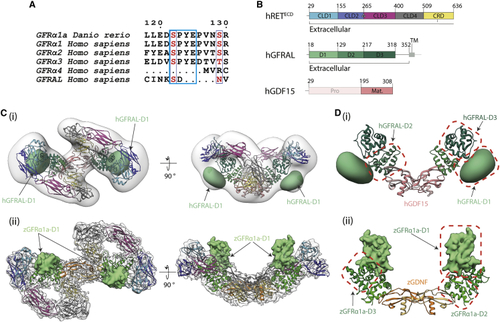
Divergent GFRα1/GFRAL co-receptor D1 domain positions within the RETECD ternary complex (A) The D1-D2 domain linker motif (SPYE), highlighted in cyan is conserved between zGFRα1a, GFRα1, GFRα2, and GFRα3. It is missing from the shorter GFRα4 that lacks a D1 domain altogether and from the divergent GFRAL. (B) Schematic diagram of human RETECD, GFRAL, and GDF15 construct boundaries used and individual domains annotated as in (C) (i) Negative stain EM envelope of a reconstituted hRETECD2-hGDF152-hGFRAL2 (hR15AL) complex docked with hR15AL (PDB: (D) Comparison of co-receptor D1 domain position and interfaces (i) GFRALD1 makes different contacts to domains D2-D3 (green), GFRALD1 shown as a 30 Å2 Gaussian volume (light green), and GDF15 (salmon). (ii) zGFRα1aD1 contacts and colored as in |
Reprinted from Structure (London, England : 1993), 29(7), Adams, S.E., Purkiss, A.G., Knowles, P.P., Nans, A., Briggs, D.C., Borg, A., Earl, C.P., Goodman, K.M., Nawrotek, A., Borg, A.J., McIntosh, P.B., Houghton, F.M., Kjær, S., McDonald, N.Q., A two-site flexible clamp mechanism for RET-GDNF-GFRα1 assembly reveals both conformational adaptation and strict geometric spacing, 694-708.e7, Copyright (2021) with permission from Elsevier. Full text @ Structure