- Title
-
Sinus venosus adaptation models prolonged cardiovascular disease and reveals insights into evolutionary transitions of the vertebrate heart
- Authors
- Gafranek, J.T., D'Aniello, E., Ravisankar, P., Thakkar, K., Vagnozzi, R.J., Lim, H.W., Salomonis, N., Waxman, J.S.
- Source
- Full text @ Nat. Commun.
|
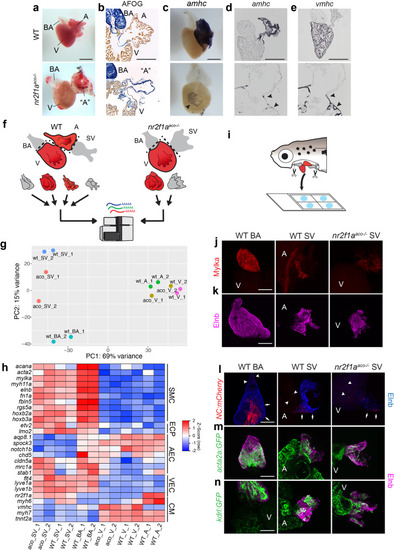
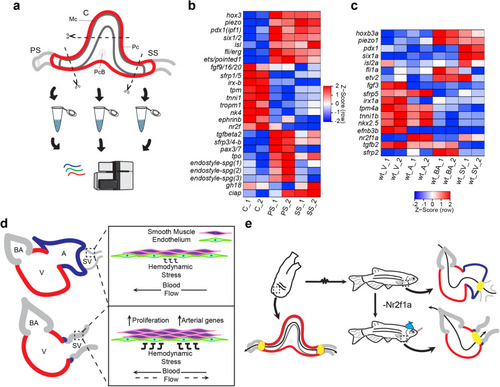
Inflow tract tissue adjacent to the venous pole of the heart tissue expands in adult EXPRESSION / LABELING:
PHENOTYPE:
|
|
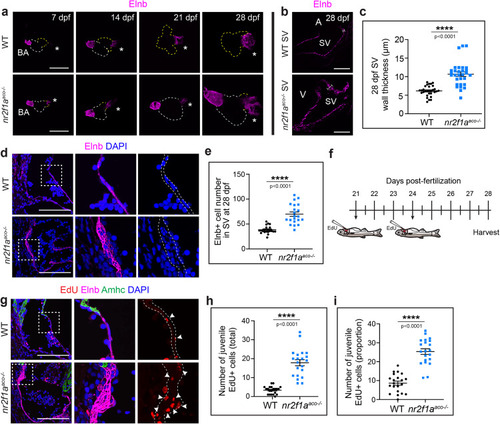
EXPRESSION / LABELING:
PHENOTYPE:
|
|
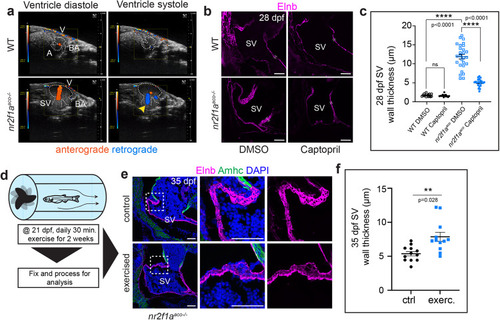
Aberrant blood flow contributes to remodeling in PHENOTYPE:
|
|
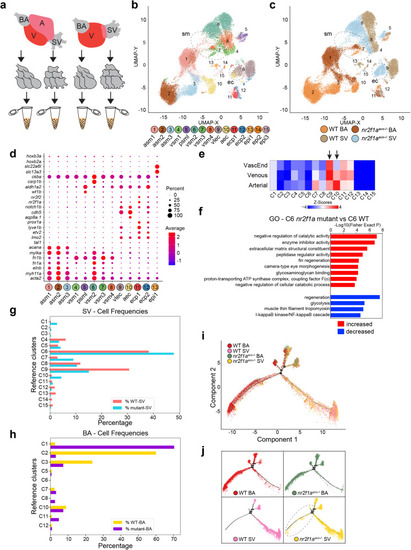
The remodeled |
|
Blood sinuses of the tunicate heart have conserved expression of genes found in the teleost BA and SV. |