- Title
-
Multiple asters organize the yolk microtubule network during dclk2-GFP zebrafish epiboly
- Authors
- Marsal, M., Bernardello, M., Gualda, E.J., Loza-Alvarez, P.
- Source
- Full text @ Sci. Rep.
|
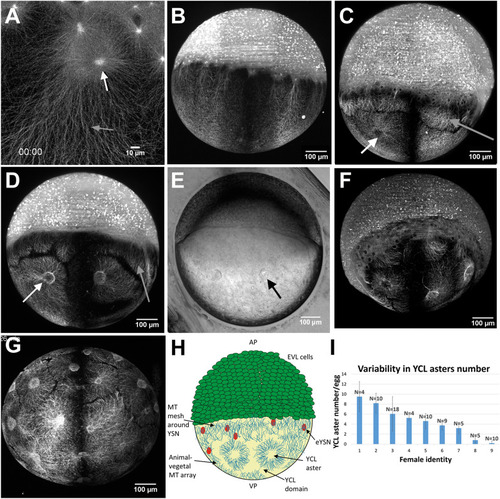
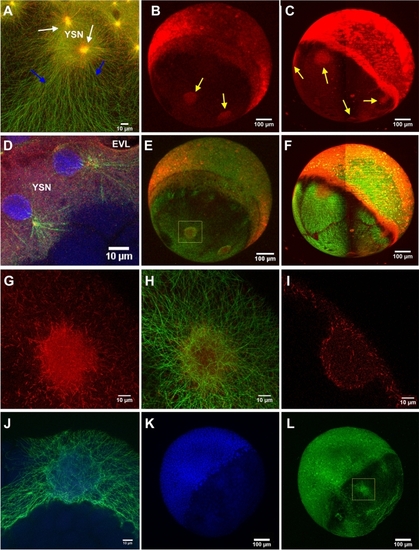
The variable presence of YCL asters in the yolk MT network of dclk2-GFP transgenic zebrafish embryos. ( |
|
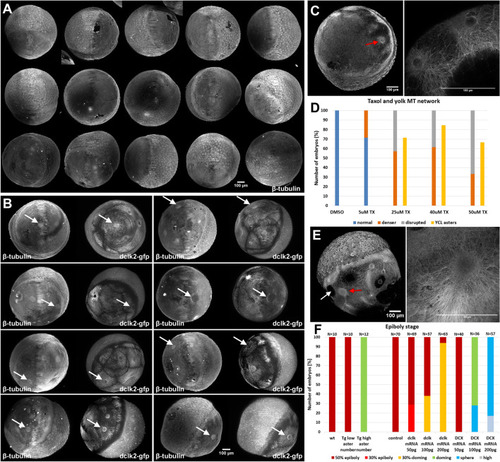
Natural and induced formation of YCL asters. ( |
|
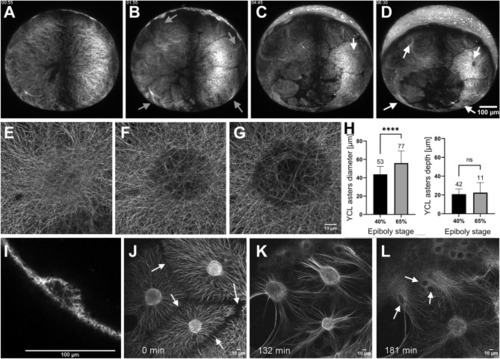
The YCL asters form and evolve throughout epiboly. ( |
|
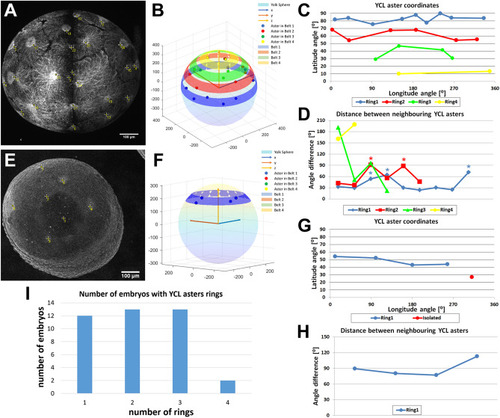
Analysis of the spatial YCL asters distribution in rings. ( |
|
MT polymerization occurs at YSL and across the YCL |
|
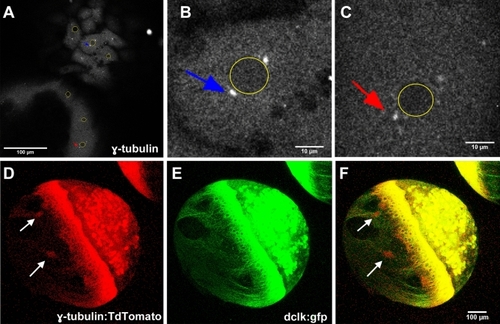
MT nucleation occurs at YSN centrosomes and YCL asters. γ-Tubulin expression can be observed through ( |