- Title
-
Knockdown of hspg2 is associated with abnormal mandibular joint formation and neural crest cell dysfunction in zebrafish
- Authors
- Castellanos, B.S., Reyes-Nava, N.G., Quintana, A.M.
- Source
- Full text @ BMC Dev. Biol.
|
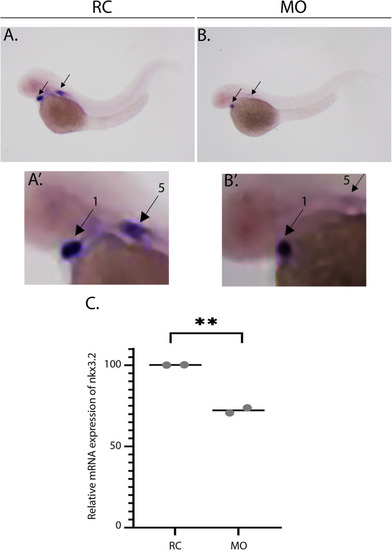
Knockdown of PHENOTYPE:
|
|
|
|
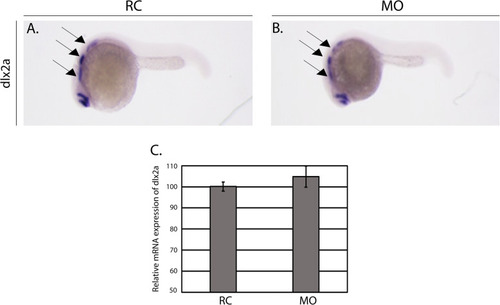
Early neural crest cell (NCC) migration and specification are normal in morphants. PHENOTYPE:
|
|
|
|
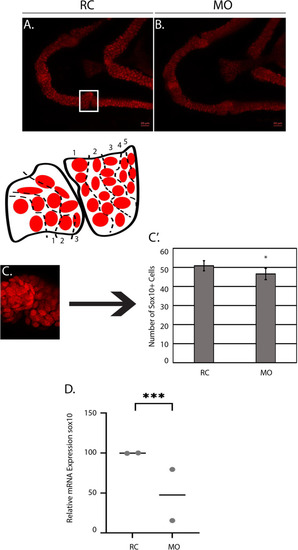
The number of Sox10+ cells is increased in morphants at 3 DPF. EXPRESSION / LABELING:
PHENOTYPE:
|
|
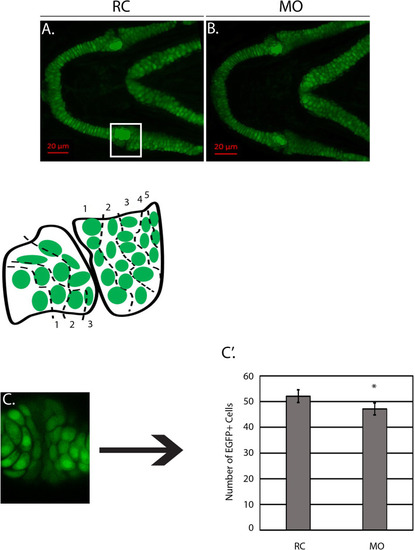
The number of Sox10+ cells is decreased in morphants at 4 DPF. |
|
The number of Col2a1a+ cells is decreased at 4 DPF. |