- Title
-
Amyloid-like Self-Assembly of a Cellular Compartment
- Authors
- Boke, E., Ruer, M., Wühr, M., Coughlin, M., Lemaitre, R., Gygi, S.P., Alberti, S., Drechsel, D., Hyman, A.A., Mitchison, T.J.
- Source
- Full text @ Cell
|
A Balbiani Body Is a Non-Membrane-Bound Compartment Packed with Membranous Organelles (A) Phase contrast image of a stage I Xenopus laevis oocyte. Bb, Balbiani body; N, nucleus or germinal vesicle. (B) Balbiani body immobilized in perfusion chambers. 2 M NaCl (first panel) or 95°C 50 mM HEPES, 100 mM KCl (pH 7.6) buffer (second panel) was perfused into the chambers. (C) Thin-section electron microscope (EM) images of isolated Balbiani bodies from stage I Xenopus oocytes. Mitochondria (dark spots), RNP particles (green arrow head), and Golgi stacks (yellow arrow head) are clearly visible. (D) Stage I oocytes were incubated in 10 μM Thioflavin T in 1× MMR for 10 min and washed twice with 1× MMR. See also Figure S1, Table S1, and Movie S1. |
|
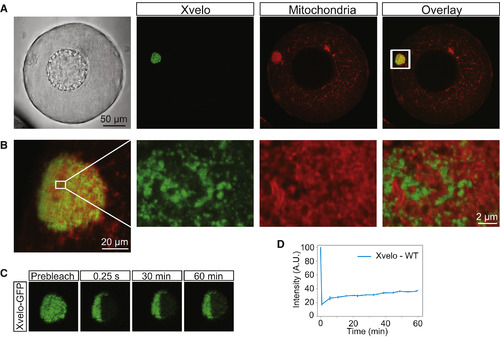
Xvelo Forms a Stable Matrix (A) mRNA encoding for Xvelo-GFP was microinjected into stage I oocytes. MitoTracker Deep Red was used to label mitochondria. Oocytes were imaged live with a laser scanning confocal microscope with a 40× water-immersion objective. (B) Magnification of the Balbiani body in (A). (C) Internal rearrangement of fluorescent Xvelo-GFP particles after half bleach over time. (D) The fluorescent recovery of the half-bleached Xvelo-GFP in the Balbiani body in (C) and two other biological replicates is shown by quantification of fluorescence in bleached region over time. Fluorescent intensity changes in the bleached region per pixel over time were plotted after it was normalized for photobleaching by using an unbleached neighboring area and background subtraction. See also Figure S2. |
|
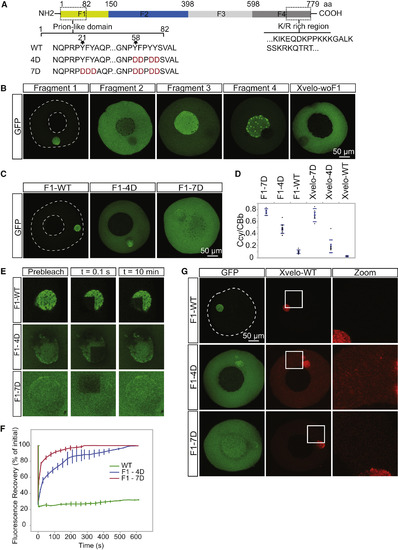
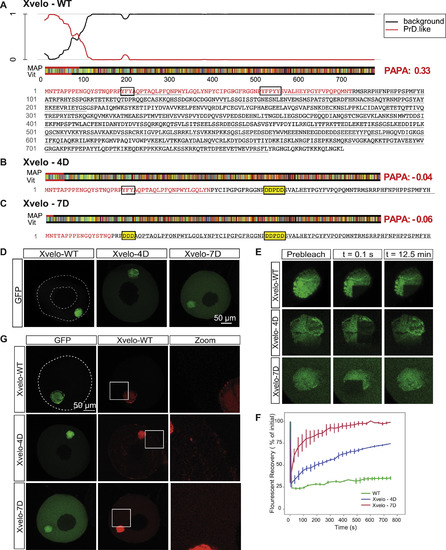
Xvelo Self-Assembly Is Dependent on Its Prion-like Domain (A) Diagram of the known structural elements of Xvelo. Prion-like domain, mutants (4D and 7D) and the fragments of Xvelo (F1–F4) are marked in the figure. (B) mRNAs encoding for Xvelo fragments shown in (A) and Xvelo without fragment 1 (Xvelo-woF1) are in vitro synthesized and micro-injected into stage I oocytes. Oocytes were imaged after overnight incubation in oocyte culture medium (OCM). (C) mRNAs encoding for wild-type and PLD mutants of fragment 1-GFP were microinjected into oocytes. Oocytes were incubated overnight and imaged. (D) Ratio of GFP concentration in the oocyte cytoplasm (Ccy) to the Balbiani body (CBb) in oocytes injected with mRNAs encoding for indicated proteins. Relative concentrations were calculated by using oocyte or the Balbiani body volume from z stacks and the fluorescent intensity of GFP. Mean values and SEs of 10 oocytes are plotted. (E) Internal rearrangement of fluorescent wild-type or mutant F1-GFP particles after photobleaching over time. (F) The fluorescent recovery of photobleached wild-type or mutant F1-GFP in Balbiani bodies in (E) and two other biological replicates for each are shown by quantification of fluorescence in bleached region over time normalized by an unbleached neighboring region. (G) mRNAs encoding for full-length Xvelo-mCherry wild-type and GFP-tagged fragments (F1-WT-GFP, F1-4D-GFP, and F1-7D-GFP) were in vitro synthesized. F1-WT-GFP or mutants were mixed with equal amounts of full-length Xvelo-mCherry mRNA and microinjected into the oocytes. After overnight incubation, the oocytes were imaged by scanning confocal microscopy. See also Figure S3. |
|
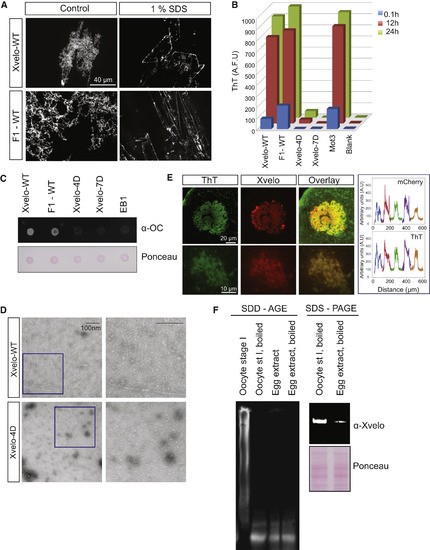
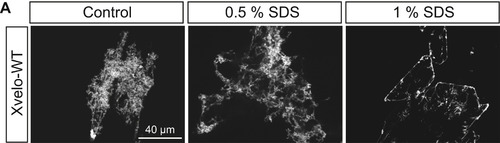
Xvelo Shows Amyloid-like Features In Vivo and In Vitro (A) SDS was added to Xvelo and F1-WT-GFP networks to a final 1% concentration, and the reactions were incubated at room temperature for 15 min. The resulting mixtures were squashed under a coverslip and imaged by a spinning-disc confocal microscope. (B) A final concentration of 5 μM of Thioflavin T was added to the wild-type, F1, and mutant network reactions at the indicated time points. Yeast prion Mot3 was used as a positive control, whereas blank was only buffer and ThT. ThT fluorescence was measured (a.u.) by a fluorescence plate reader. (C) 1 μg RFP-tagged wild-type, F1, and mutant recombinant proteins were dot-blotted on a nitrocellulose membrane and assayed for reactivity with α-amyloid fibril OC. EB1-RFP was used as a negative control. (D) Negative stain electron microscopy images of the untagged Xvelo-WT and Xvelo-4D self-assembly reactions (scale bars, 100 nm). (E) Stage I oocytes were injected with mRNA coding for Xvelo-mCherry and incubated overnight. The oocytes were incubated in 10 μM ThT, washed twice, and imaged by confocal microscopy. Bottom: zoomed in images. Line scans showing the co-localization of Xvelo-mCherry and ThT stain from five Balbiani bodies were plotted. Each color represents the line scan of a different Balbiani body. We speculate that the outer rim Xvelo-mCherry signal belongs to the newly translated Xvelo-mCherry protein that has just started to form a new, immature matrix and does not yet stain with ThT. (F) SDD-AGE detects SDS-resistant Xvelo aggregates in vivo. Equal amounts of cytoplasmic extracts of stage I oocytes and mature eggs were loaded onto SDS-PAGE. A five times more amount of egg extracts was loaded for SDD-AGE gels to make Xvelo concentrations comparable between the oocyte and egg extract lanes. Xvelo was detected by an anti-Xvelo antibody. See also Figure S5. |
|
Xvelo Has Unique Properties for Forming a Stable Matrix (A) mRNAs encoding for GFP-tagged hnRNPA1, CPEB3, Tia1, Dazap1, FUS, and FUS156E were in vitro synthesized and microinjected into the oocytes. After overnight incubation, the oocytes were imaged by laser scanning confocal microscopy. (B) mRNAs encoding for Xvelo-mCherry and GFP-tagged Bucky ball (Buc), FUS, and the PLD-swap versions of Xvelo, in which the PLD of Xvelo was replaced either by the PLD of Bucky ball (BucPLDXvelo) or the PLD of FUS (FUSPLDXvelo), were injected into the oocytes at equal concentrations and imaged after overnight incubation. (C) Internal rearrangement of fluorescent Bucky ball-GFP (Buc), and PLD-swap versions of Xvelo, Buc(PLD)Xvelo-GFP and FUS(PLD)Xvelo-GFP, after photobleaching over time. (D) The fluorescent recovery of photobleached constructs in Balbiani bodies in (C), as well as Xvelo-WT and two other biological replicates for each, is shown by quantification of fluorescence in a bleached region over time normalized by an unbleached neighboring region. See also >Figure S6. |
|
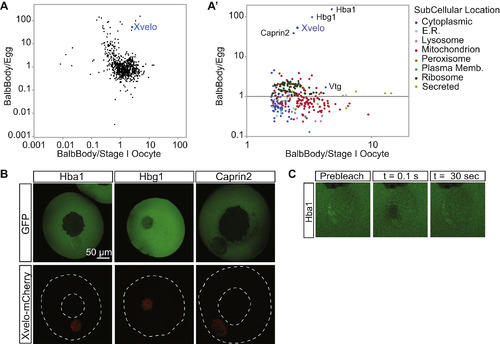
Xvelo Is Enriched in Balbiani Bodies, Related to Figure 1 (A) Isolated Balbiani bodies were TMT labeled as well as laid eggs or stage I oocytes and analyzed by quantitative mass spectrometry. Shown here are the ratios of Balbiani body/egg or Balbiani body/stage I oocyte for each identified protein plotted on a log scale. The protein composition of freshly dissected Balbiani bodies was compared with either whole stage I oocytes, or laid eggs. As a technical caveat, differences in solubility of a protein in Guanidine hydrochloride between oocytes and eggs could give rise to systematic error in ratio estimates. (A′) The enriched proteins in Balbiani body/ stage I oocyte are enlarged and color coded to reveal their subcellular localization. Vtg is vitellogenin, the yolk protein; Hba1 and Hbg1 are hemoglobin subunits, and Caprin2 is an RNA binding protein. When compared to whole stage I oocytes, the most enriched proteins in Balbiani bodies were mitochondrial proteins, as well as other proteins residing in organelles, as expected. This finding was consistent with EM views, where most of the organelles in a stage I oocyte are concentrated in the Balbiani body (Figure 1A), while in eggs they are dispersed. Therefore, to identify enriched non-organelle proteins, we compared Balbiani bodies to laid eggs, where the Balbiani body is dispersed (Table S1). (B) Among the enriched proteins in the Balbiani bodies, only Xvelo localized to the Balbiani body whereas the fetal hemoglobin subunits Hba1 and Hbg1, and Caprin2 remained mostly cytoplasmic in stage I oocytes. This could be explained by a lack of binding partners and targeting sequences to the Balbiani body for all three of these proteins: Hemoglobins need their cofactor, heme, for proper folding and function. Oocytes are rich in hemoglobins (Peshkin et al., 2015) and a lack of accessible heme would interfere with the folding of newly synthesized hemoglobin subunits. Caprin2 is an RNA binding protein (Shiina and Tokunaga, 2010), and at the stage when the Balbiani body is already formed, supposedly all of its related RNAs would already be bound to the endogenous Caprin2 that is present in the Balbiani body. In the absence of a targeting signal or non-specific binding potential, these proteins are not expected to localize to the Balbiani body. (C) The Balbiani body-localized Hba1 has a fast recovery time after photo-bleaching, suggesting it is not part of the structural matrix in the Balbiani body. |
|
Xvelo Has a Prion-like Domain Predicted by PLAAC and PAPA, and Full-Length Mutants of Xvelo Recapitulated F1 Fragment Kinetics, Related to Figure 3 (A) Xvelo has an N-terminal prion-like domain predicted by PLAAC, and PAPA prion prediction programs. PLAAC results are shown here, and PAPA scores are highlighted in red at the right handside (Toombs et al., 2012 ; Lancaster et al., 2014). The aminoacid sequence of the protein is color coded according to Lancaster et al., 2014. The aromatic residues which are mutated are boxed in for ease of visualization. PrD.like: prion-like domain. Vit and Map correspond to the maximum length of consecutive PrD state in Viterbi or MAP parses. (B) Mutating the aromatic residues in the prion-like domain of Xvelo to charged residues, YFPYY into DDPDD, stopped Xvelo score positive in PAPA prion detection algorithm. 4D mutant scored –0.04 in PAPA, and the aggregation-prone region detected by PLAAC decreased more than half in length. (C) Additional mutations of aromatic residues in (B) to charged residues, YFY to DDD, further eliminated the detection of Xvelo by prion-prediction algorithms. The PAPA score decreased to –0.059, and PLAAC detected few aminoacids at the N-terminal as aggregation-prone. (D) Full-length Xvelo-GFP, Xvelo-4D-GFP and Xvelo-7D-GFP mRNAs were microinjected into oocytes, incubated overnight in OCM, and imaged by scanning confocal microscopy. Note the 4D mutation has a stronger effect on the localization of the F1 fragment than the full-length protein (Figure 3D). (E) A region corresponding to a quarter of a Balbiani body was photobleached in the oocytes shown in (D), and the fluorescence recovery of GFP was monitored in every 30 s for 12.5 min. (F) Quantification of the oocytes photobleached in (E) and two other biological replicates. SEs were plotted. (G) mRNAs encoding for full-length Xvelo-mCherry, Xvelo-GFP, Xvelo-4D-GFP and Xvelo-7D-GFP were co-injected into oocytes, and imaged after overnight incubation. |
|
Xvelo Networks Consist of SDS-Resistant Backbones Attached to More SDS-Soluble Side Chains, Related to Figure 5 (A) Once incubated at a lower SDS concentration (0.5%), the side chains also are SDS resistant. Xvelo network self-assembly reactions were initiated by lowering the arginine concentration to 30mM (in 50mM HEPES, 100mM KCl [pH] 7.6) and 1 or 0.5% SDS was added to these networks. After 30 min incubation at room temperature, the networks were imaged with a spinning disc confocal microscope. |
|
Xvelo Has Unique Properties in Forming a Stable Matrix In Vivo, and Xvelo Self-Assembly Is Specific In Vitro, Related to Figure 6 (A) Internal rearrangement of fluorescent Tia1-GFP and GFP-Dazap1, as well as Xvelo-WT-GFP after photobleaching over time. (B) The fluorescent recovery of photobleached fluorescent proteins in (A) and two other biological replicates for each are shown by quantification of fluorescence in bleached region over time normalized by an unbleached neighboring region. (C) Schematic representations of Xvelo, Bucky ball (Buc), FUS and the chimeric proteins Buc(PLD)Xvelo and FUS(PLD)Xvelo after the domain swap. (D) FUS-WT-GFP, FUS-156E-GFP and Xvelo-WT-RFP were mixed in a high salt, high arginine buffer (500 mM KCl, 300 mM Arginine, 50 mM HEPES, [pH 7.6]). The mixture is then diluted to 100 mM KCl, 30mM Arginine (50 mM HEPES, [pH 7.6]) to initiate the self-assembly/ aggregation reactions. FUS-WT-GFP and FUS-G156E-GFP control reactions were initiated by diluting down the KCL concentration to 100 mM. Note the FUS-WT droplets were smaller in the presence of Arginine. |
Reprinted from Cell, 166, Boke, E., Ruer, M., Wühr, M., Coughlin, M., Lemaitre, R., Gygi, S.P., Alberti, S., Drechsel, D., Hyman, A.A., Mitchison, T.J., Amyloid-like Self-Assembly of a Cellular Compartment, 637-50, Copyright (2016) with permission from Elsevier. Full text @ Cell