Fig. S1
|
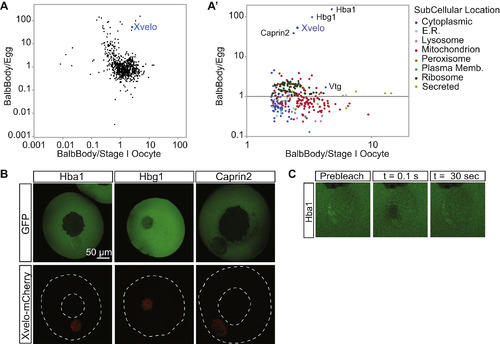
Xvelo Is Enriched in Balbiani Bodies, Related to Figure 1 (A) Isolated Balbiani bodies were TMT labeled as well as laid eggs or stage I oocytes and analyzed by quantitative mass spectrometry. Shown here are the ratios of Balbiani body/egg or Balbiani body/stage I oocyte for each identified protein plotted on a log scale. The protein composition of freshly dissected Balbiani bodies was compared with either whole stage I oocytes, or laid eggs. As a technical caveat, differences in solubility of a protein in Guanidine hydrochloride between oocytes and eggs could give rise to systematic error in ratio estimates. (A′) The enriched proteins in Balbiani body/ stage I oocyte are enlarged and color coded to reveal their subcellular localization. Vtg is vitellogenin, the yolk protein; Hba1 and Hbg1 are hemoglobin subunits, and Caprin2 is an RNA binding protein. When compared to whole stage I oocytes, the most enriched proteins in Balbiani bodies were mitochondrial proteins, as well as other proteins residing in organelles, as expected. This finding was consistent with EM views, where most of the organelles in a stage I oocyte are concentrated in the Balbiani body (Figure 1A), while in eggs they are dispersed. Therefore, to identify enriched non-organelle proteins, we compared Balbiani bodies to laid eggs, where the Balbiani body is dispersed (Table S1). (B) Among the enriched proteins in the Balbiani bodies, only Xvelo localized to the Balbiani body whereas the fetal hemoglobin subunits Hba1 and Hbg1, and Caprin2 remained mostly cytoplasmic in stage I oocytes. This could be explained by a lack of binding partners and targeting sequences to the Balbiani body for all three of these proteins: Hemoglobins need their cofactor, heme, for proper folding and function. Oocytes are rich in hemoglobins (Peshkin et al., 2015) and a lack of accessible heme would interfere with the folding of newly synthesized hemoglobin subunits. Caprin2 is an RNA binding protein (Shiina and Tokunaga, 2010), and at the stage when the Balbiani body is already formed, supposedly all of its related RNAs would already be bound to the endogenous Caprin2 that is present in the Balbiani body. In the absence of a targeting signal or non-specific binding potential, these proteins are not expected to localize to the Balbiani body. (C) The Balbiani body-localized Hba1 has a fast recovery time after photo-bleaching, suggesting it is not part of the structural matrix in the Balbiani body. |
Reprinted from Cell, 166, Boke, E., Ruer, M., Wühr, M., Coughlin, M., Lemaitre, R., Gygi, S.P., Alberti, S., Drechsel, D., Hyman, A.A., Mitchison, T.J., Amyloid-like Self-Assembly of a Cellular Compartment, 637-50, Copyright (2016) with permission from Elsevier. Full text @ Cell