- Title
-
Position dependence of hemiray morphogenesis during tail fin regeneration in Danio rerio
- Authors
- Murciano, C., Pérez-Claros, J., Smith, A., Avaron, F., Fernández, T.D., Durán, I., Ruiz-Sánchez, J., García, F., Becerra, J., Akimenko, M.A., and Marí-Beffa, M.
- Source
- Full text @ Dev. Biol.
|
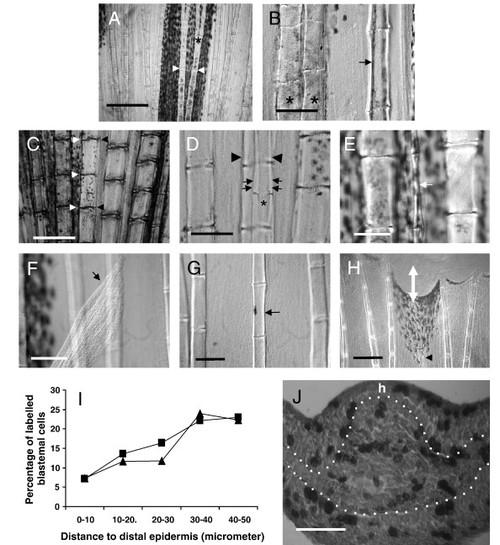
The hemiray, or a hemiray fragment, may regenerate in the caudal fin of Danio rerio. (A) The hemirays of a given ray are symmetrical. Segment joints (arrowheads) and bifurcations (asterisk) occur in exactly the same place in both hemirays. (B) After operation A, the hemiray regenerates showing a much thinner profile (arrow) and do not bifurcate. Compare the width of the regenerated hemiray (arrow) with the neighboring ray (left). Control ray regenerate normally bifurcate (asterisk) whereas hemirays regenerates do not. (C) After operation B, a thin H3 regenerate (small arrows) is obtained. Loss of coordination between segment joints occurs between regenerating hemiray (asterisk) and contralateral extant hemiray (arrowheads). (D) After operation B, partial hemiregenerates (white arrow) can also be obtained. Hemiray width was extremely narrow in this case and the complete original size was not achieved. The lengths of the segments, however, are similar to those in the neighboring rays. (E) After operation C, the hemiray may regenerate outside of the fin (arrow). In these cases, the size of the neighboring rays was never achieved after 1 month. (F) A detail of an H1 regenerate growing inside the fin after operation C. The width of the hemiray (arrow) is thinner than control neighboring rays. (G) A detail of distal regions of a regenerate after cut following operation C. Observe the hemiray (arrowhead) nearly reaches the complete size of neighboring rays (white double arrow shows non-regenerated distance). (H) Graphic showing the average percentage of BrdU-labeled blastemal cells against distance to distal epidermis (μm). ▲: hemiray blastemas, •: ray blastemas. Observe that distal 10 μm blastemal cells rarely incorporate BrdU in both hemiray and ray blastema. (I) Transversal section of a proliferating hemiblastema after operation A. BrdU is incorporated to dividing cells in both blastemal mesenchyme and epidermis. h shows the side in which the hemiray regenerate. Scale bar represents 500 μm (A), 300 μm (G), 125 μm (C, F), 100 μm (B, D, E) and 50 μm (I). EXPRESSION / LABELING:
|
|
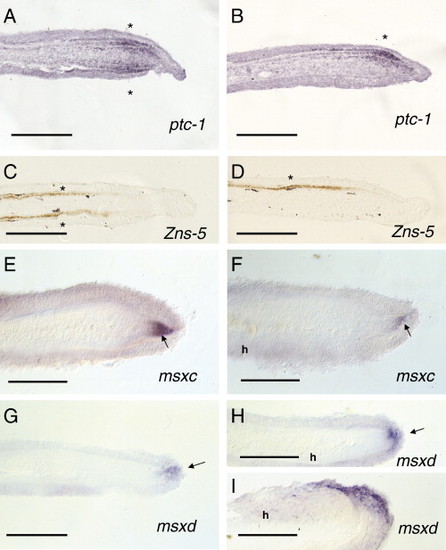
In situ hybridization of fin blastema 4 or 5 days post-operation B. (A) ptc-1 expression is restricted to scleroblasts in both sides (asterisk) in control regenerating blastemas. (B) ptc-1 expression is observed in the scleroblasts regenerating the hemiray side (asterisk) in the experimental blastema. (C) Zns-5 expression is detected in both precursors of scleroblasts at both blastema sides (asterisks) during ray regeneration. (D) Zns-5 expression is exclusively detected in the regenerating hemiblastema at the level of both precursors of scleroblasts and scleroblasts (asterisk). (E, F) msxc expression is observed at a DMB (arrow in panel E) or DMH (arrow in panel F) in both control (E) and hemiray (h) blastemas. (G, H) msxd expression is restricted to the distalmost epidermis (arrow) covering the DMB (G) and DMH (H). (I) msxd expression is lateralized in the distal epidermis of a regenerating hemiblastema (H, I). h represents the side carrying a regenerating hemiray) Scale bar represents 100 μm (A–I). |
|
Regenerated recombinant rays with hemirays from different origins can be obtained after hemiray fragment grafting and cut at the level of the graft. (A) The hemiray graft was fixed by a knobbed loop of a very thin surgical thread. (B) After cut, homotopical graft of an H3 fragment of the caudal fin leads to a symmetrical normal R3. Inset shows the homotopical graft proximal to the level of the cut. The black arrowhead shows the thread used for the graft. The white arrowhead shows the grafted fragment. (C) Transversal section of a recombinant H1 → H9 ray in which H1 was labeled with DiI. DiI-labeled cells were observed in one side of the ray blastema (H1). However, some positive cells were also observed in the contralateral side (H9) within the blastemal mesenchyme. Epidermal cells were never stained in the contralateral side (arrowheads). (D) Distal and proximal (inset) detail of an H1 → H9 recombinant regenerate. The ray bifurcates (arrowheads) and shows a pattern and size similar to the neighboring rays. The inset includes the grafted fragment (arrowhead) remaining at the stump. White cells (small, rounded and orange in color) are pigment cells characteristic of R1 rays (Johnson et al., 1995) and are observed in the grafted region. (E) Intermediate region of the H1 → H9 regenerate showing bifurcating (asterisk) contralateral hemirays with a symmetrical pattern. (F) Distal and proximal (inset) detail of an H9 → H3 regenerate. Observe that the regenerate (arrowhead) shows a size similar to the neighboring rays. The inset includes the stump of the regenerate showing the grafted fragment. The black arrowhead shows the thread used for the graft. The white arrowhead shows the grafted fragment. (G) Detail of an intermediate position of an H9 → H3 regenerate (arrowhead) showing hemirays with a symmetrical pattern. R4 and R2 means rays 4 and 2 in any lobe. (H) Distal and proximal (inset) details of an H3p → H3d graft regenerate. Observe the recombinant ray bifurcates (arrowheads) at the level of the neighboring R4 ray. The inset includes the remaining graft fragment at the level of the cut. (I) A detail of a medial region of an H3d-H3p recombinant ray regenerate. Observe the symmetrical pattern that shows the recombinant regenerate. The black arrowhead shows the grafted fragment. Scale bar represents 300 μm (D, E), 125 μm (F, G and in inset of panels D, F, H, and I), 100 μm (B, C, H, I and in inset of panel B) and 50 μm (A). |
|
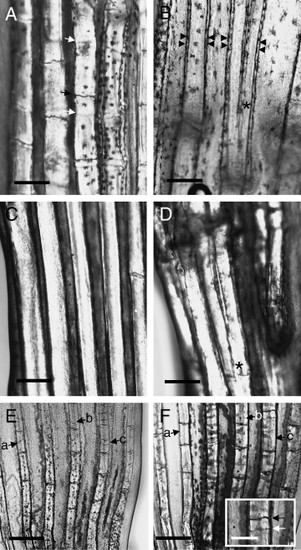
Phenotype of developing and regenerating caudal fins of alfty86d mutant fishes. (A) Developing rays of the caudal of a 30.5-mm standard length (S.L.) alfty86d fish. Observe that some segmentation processes (black arrow) are not coincident in both contralateral hemirays (white arrows). (B) Developing rays of the caudal fin of a 27-mm S.L. alfty86d fish. Bifurcation processes (asterisk) may not occur in all the rays but they are symmetrical in both hemirays. Rays profile are shown with double arrowheads. (C) Developing ray of the caudal fin of a 27.5-mm S.L. alfty86d fish, showing a strong phenotype. Segmentation is almost absent in the complete ray. (D) Bifurcation (asterisk) in a 26-mm S.L. strong phenotype fish does not occur in all the rays. (E) Initial regenerating rays (14 days post-operation) in the caudal fin of an alfty86d mutant 27 mm S.L. adult fish. Observe that many partial segmentation processes (a–c arrows) occur in different positions irrespective of the contralateral pattern. (F) Fifty-four days post-operation, in the same regenerate region as the previous figure many partial segmentation processes (a–c arrows) disappeared as the lepidotrichia grows in thickness. Only the initially most conspicuous ones remain. Inset shows loss of hemi-joint registration (black vs. white arrows). Scale bar represents 250 μm (A–D and inset in panel F); 500 μm (E, F). |
Reprinted from Developmental Biology, 312(1), Murciano, C., Pérez-Claros, J., Smith, A., Avaron, F., Fernández, T.D., Durán, I., Ruiz-Sánchez, J., García, F., Becerra, J., Akimenko, M.A., and Marí-Beffa, M., Position dependence of hemiray morphogenesis during tail fin regeneration in Danio rerio, 272-283, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.