- Title
-
nanos1 is required to maintain oocyte production in adult zebrafish
- Authors
- Draper, B.W., McCallum, C.M., and Moens, C.B.
- Source
- Full text @ Dev. Biol.
|
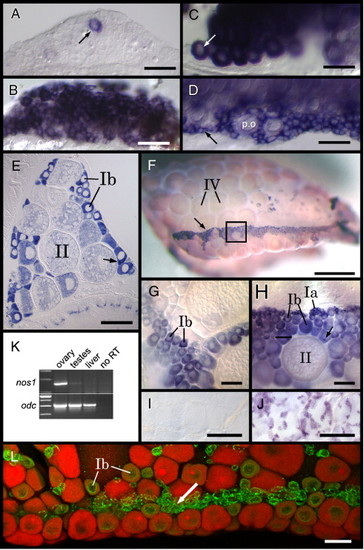
nanos1 is expressed in adult female germ cells. RNA in situ hybridization reveals that at 21 dpf (A and B) and 35 dpf (C and D). nos1 expression (A and C) appears to be restricted to perinuclear stage oocytes (arrows) as compared to vasa (B and D) that appears to be expressed in both perinuclear stage oocytes (p.o.) as well as germ cells that are less than 20 μm in diameter (arrow in panel D). (E) RNA in situ hybridization of a transverse section through a 2-month-old female zebrafish reveals that nos1 is expressed at high levels in early stage oocytes (oriented dorsal up). In stage Ib oocytes, nos1 transcripts are enriched in a spherical cytoplasmic structure that resembles the Balbiani body (arrow). (F) In adult ovaries, vasa expression reveals that early stage oocytes localize to a ventral zone on the ovarian surface (arrow; ovary is oriented with anterior to the left and dorsal up). (H) Higher magnification of region boxed in panel F reveals an overall organization of early stage oocytes within this ventral zone, with stage Ia oocytes located dorsal to stage Ib oocytes. Similar to nos1, vasa RNA is also enriched in spherical cytoplasmic structures within stage Ib oocytes (arrow). (G) nos1 is highly expressed in stage Ib oocytes, but is either not expressed in clusters of stage Ia oocytes that localize to the ventral zone, or is expressed at levels below our ability to detect (compare G and H). nos1 expression cannot be detected in testes by in situ hybridization (I) while vasa expression can be readily detected in developing spermatocytes (J). (K) RT-PCR analysis confirms that nos1 expression in adults is female-specific. ornithine decarboxylase (odc) expression analysis is included as a positive control. (L) A confocal image of an ovary double-labeled for Vasa protein (green) and nos1 RNA (red) shows that nos1 and Vasa co-localize in early stage oocytes (Ib) but not in the smallest (< 20 μm) Vasa-positive germ cells (arrow). Scale bars: 50 μm in panels A–D, L; 200 μm in panels E, I and J; 1 mm in panel F; 100 μm in panles E and F. EXPRESSION / LABELING:
|
|
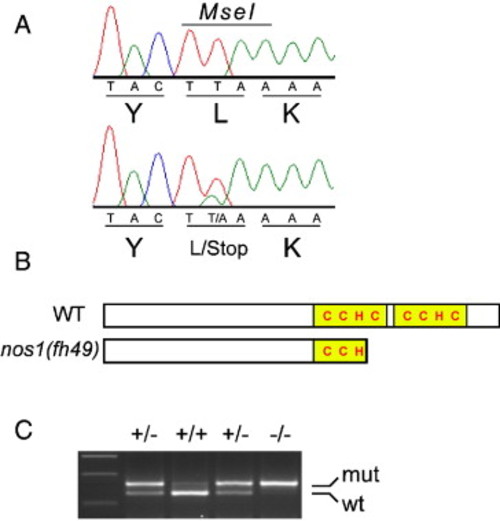
The nos1(fh49) mutant allele encodes a truncated form of the protein. (A) Sequence traces of nos1 from wild-type (top) and nos1(fh49) heterozygotes reveal that nos1(fh49) is a point mutation that converts the Leu(110) codon into a stop codon. The mutation disrupts an MseI restriction enzyme site present in wild-type. (B) Schematic of the predicted wild-type and mutant Nos1 protein structures. The CCHC zinc finger RNA-binding domains are shown in yellow. (C) PCR-based genotyping assay utilizing the MseI restriction site polymorphism. nos1(fh49) heterozygotes have both wild-type (lower) and mutant (upper) bands. |
|
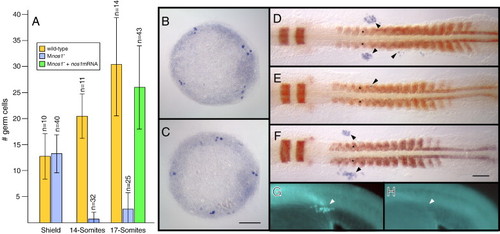
Maternal nos1 is required for primordial germ cell survival. (A) Numbers of germ cells in embryos at the stages identified on the X axis as assayed by in situ hybridization using a vasa probe. Shield and 14-somite stage embryos were derived from a cross between nos1-/- females and nos1+/+ males, while 17-somite stage embryos were derived from a cross between nos1-/- parents. Standard deviations and numbers of embryos analyzed for each class are indicated. In legend, Mnos1- indicates embryos derived from nos1-/- females. PGC localization in representative shield-stage embryos derived from nos1+/+ females (B) or nos1-/- females (C) as revealed by vasa in situ hybridization (blue). PGC localization in representative 17-somite stage embryos stained for myod and egr2b in red, and vasa in blue, derived from nos1+/+ females (D), nos1-/- females (E) or nos1-/- females injected with 200 pg synthetic nos1 mRNA (F). Arrows point to correctly localized vasa expressing PGCs, while arrowheads indicate ectopically localized PGCs. Germ cell development in Mnos1 embryos can be rescued by injection of nos1(wt) RNA (G; n = 12/12) but not nos1(fh49) mutant RNA (H; n = 0/23) at the one-cell stage, as assayed in a 24 hpf embryo. Arrow in panel G points to EGFP expressing germ cells (see Materials and methods). Scale bars: 100 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
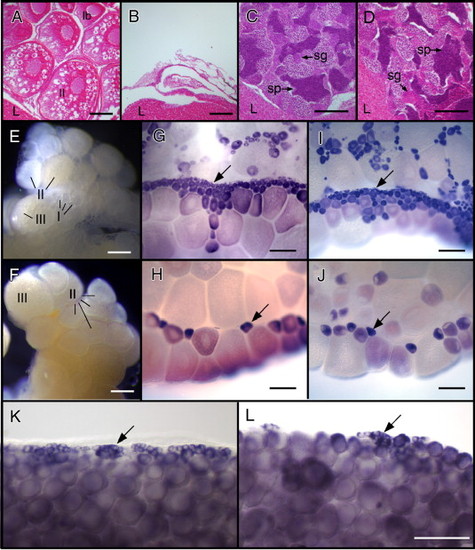
Zygotic nos1 is required for adult germline maintenance. Histology of 6-month-old females (A and B) and male (C and D) gonads. (A) Wild-type ovaries contain oocytes (stages indicated). (B) nos1 mutant females do not contain oocytes. The testes of wild-type (C) and nos1 mutants (D) are indistinguishable. Three-month-old wild-type (E, G and I) and nos1 mutant (F, H and J) ovaries freshly isolated (E and F) or stained for vasa (G and H) or ziwi (I and J) RNA. Wild-type ovaries contain numerous stage I oocytes, visualized as small (7–140 μm diameter) clear cells on the surface of the ovary (E) or as small vasa RNA+ (G) and ziwi RNA+ (I) cells (arrows). In contrast, nos1 mutant ovaries contain very few stage I oocytes (F, H, J; arrows in panels H and J). RNA in situ hybridization to detect vasa RNA reveals that 35 dpf nos1 mutant ovaries (L) contain fewer < 20 μm germ cells (arrow) than wild-type ovaries (K). sg, spermatogonia; sp, sperm; l, liver. Scale bars: 100 μm in panels A–D, K, L; 200 μm panels in E–J. EXPRESSION / LABELING:
PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 305(2), Draper, B.W., McCallum, C.M., and Moens, C.B., nanos1 is required to maintain oocyte production in adult zebrafish, 589-598, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.