- Title
-
Pseudouridylation defect due to DKC1 and NOP10 mutations causes nephrotic syndrome with cataracts, hearing impairment, and enterocolitis
- Authors
- Balogh, E., Chandler, J.C., Varga, M., Tahoun, M., Menyhárd, D.K., Schay, G., Goncalves, T., Hamar, R., Légrádi, R., Szekeres, Á., Gribouval, O., Kleta, R., Stanescu, H., Bockenhauer, D., Kerti, A., Williams, H., Kinsler, V., Di, W.L., Curtis, D., Kolatsi-Joannou, M., Hammid, H., Szőcs, A., Perczel, K., Maka, E., Toldi, G., Sava, F., Arrondel, C., Kardos, M., Fintha, A., Hossain, A., D'Arco, F., Kaliakatsos, M., Koeglmeier, J., Mifsud, W., Moosajee, M., Faro, A., Jávorszky, E., Rudas, G., Saied, M.H., Marzouk, S., Kelen, K., Götze, J., Reusz, G., Tulassay, T., Dragon, F., Mollet, G., Motameny, S., Thiele, H., Dorval, G., Nürnberg, P., Perczel, A., Szabó, A.J., Long, D.A., Tomita, K., Antignac, C., Waters, A.M., Tory, K.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
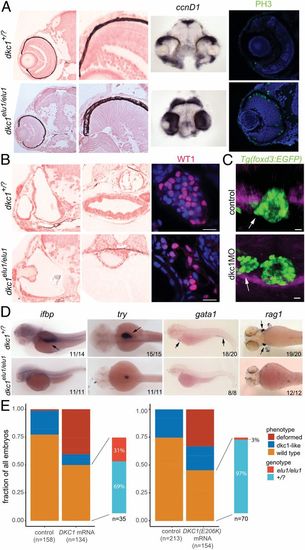
The phenotype of dkc1elu1/elu1 larvae recapitulates the human phenotype. (A) Histological analysis of dkc1elu1/elu1 mutant larvae shows microphthalmia and cataracts. Both the eyes and the optic tectum of the mutants are abnormal and contain a high prevalence of cells with neuroepithelial character. Expression of cell-cycle markers ccnD1 and PH3 in the retinae and the tecta of 2-dpf and 3-dpf larvae, respectively, can be observed throughout these tissues instead of being restricted to the proliferative regions of the ciliary marginal zone and the mediolateral edges, suggesting a defective cell cycle. (All pictures show coronal sections.) (B) Further histological analysis shows 1) deformed semicircular canals, 2) undifferentiated gut, and 3) hypoplastic pronephros with a reduced number of Wt1-positive podocytes in the mutant animals. (Scale bars: 10 μm.) (C) When Dkc1 function is abrogated in Tg(foxd3:EGFP) animals using a synthetic MO oligo, parapineal migration is impaired, and the pineal–parapineal complex appears immature at 3 dpf. (White arrows denote the parapineal.) (D) Markers of tissue differentiation demonstrate a lack of differentiation in the intestines (ifbp), pancreas (try), and the major blood lineages (gata1 and rag1). (Black arrows denote area of expression.) (E) Injection of 1) human WT DKC1 mRNA resulted in phenotypic rescue of the mutant larvae, as shown by the genotyping of larvae showing a WT phenotype. In contrast, injection of 2) human Glu206Lys DKC1 mRNA elicited a much milder rescue, demonstrating the hypomorphic nature of this allele. EXPRESSION / LABELING:
PHENOTYPE:
|
|
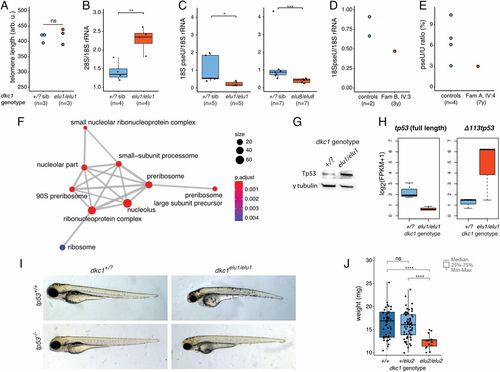
Ribosomal dysfunction in dkc1 zebrafish mutants due to defective pseudouridylation. (A) Telomere length is normal in dkc1elu1/elu1 larvae at 4 dpf as measured by flow-FISH (n = 3 pooled samples of 10 larvae each, P = 0.7). Arb. u., arbitrary units; ns, not significant. (B) The 28S/18S rRNA ratio is increased in 4-dpf dkc1elu1/elu1 larvae, suggesting impaired 18S rRNA processing (**P = 0.0033). (C and D) Immuno-Northern blot demonstrates a reduced pseudouridylation of 18S rRNA in dkc1elu1/elu1, dkc1elu8/elu8 4-dpf larvae (+/? vs. elu1/elu1: *P = 0.016, +/? vs. elu8/elu8: ***P = 0.00058) (C) and in the leukocytes of patient FamB IV:3 (D). (+/?: heterozygous or homozygous WT fish.) (E) The female with skewed X-inactivation shows a decreased pseudouridine (pseU)/U ratio in the leukocytes, as determined by HPLC-MS. (F) Gene Ontology analysis of differentially regulated genes from 36 hpf dkc1elu1/elu1 larvae demonstrates an up-regulation of genes associated with ribosome assembly and function. Size of the circles indicates the number of genes associated with certain terms, and color indicates the level of enrichment: red indicates high enrichment, and blue indicates low. P.adjust, adjusted P. (G) Western blot suggests the stabilization of Tp53 in the affected cells. (H) Transcriptomic analysis shows that the truncated, antiapoptotic tp53 isoform (Δ 113p53) is up-regulated in mutants, while the canonical, full-length, proapoptotic isoform shows decreased expression; measured as fragments per kilobase of exon model per million reads mapped. (I) The phenotype of the dkc1elu1/elu1 zebrafish mutants is Tp53-independent, as it is not rescued on a tp53− background. (J) Homozygous carriers of the missense (c.567_568insGTG) hypomorphic allele (dkc1elu2/elu2) are viable, but show significant growth retardation compared with their siblings (n = 130) (+/+ vs. elu2/elu2: ****P = 1.9 × 10−9; +/elu2 vs. elu2/elu2: ****P = 1.6 × 10−9). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|