- Title
-
G protein-coupled receptor GPR182 negatively regulates sprouting angiogenesis via modulating CXCL12-CXCR4 axis signaling
- Authors
- Chen, C., Liu, W., Yuan, F., Wang, X., Xu, X., Ling, C.C., Ge, X., Shen, X., Li, B., Shen, Y., Liu, D.
- Source
- Full text @ Angiogenesis
|
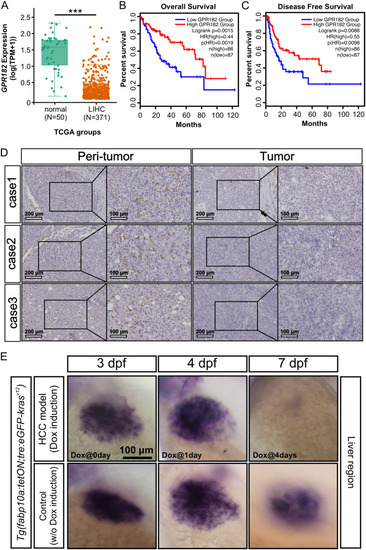
Downregulation of |
|
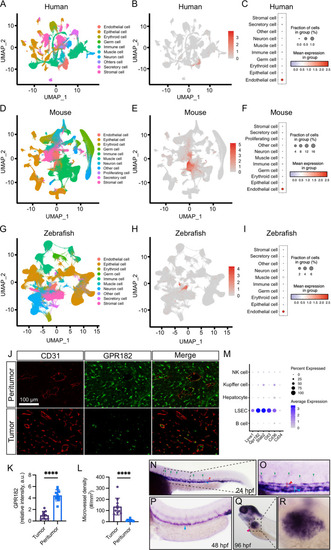
Comparative expression of GPR182 across species. |
|
Gpr182 deficiency promotes sprouting angiogenesis in zebrafish embryos. |
|
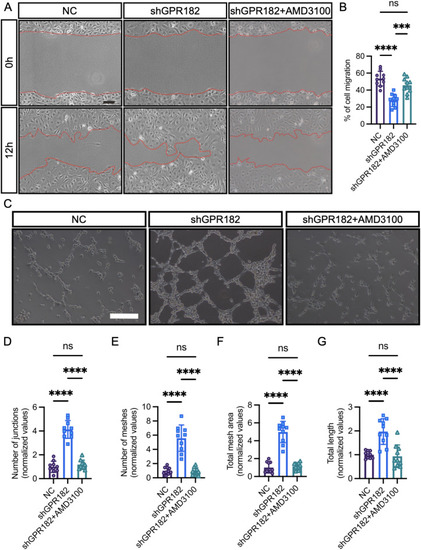
Loss of GPR182 promotes EC migration and tube formation. |
|
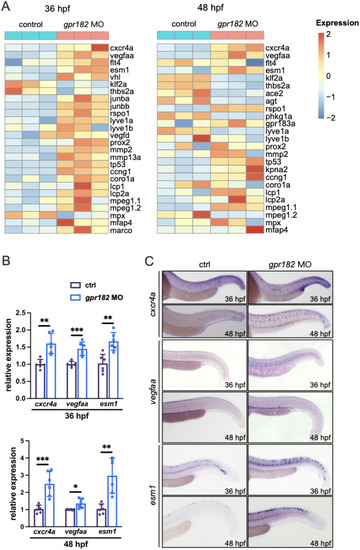
Whole-genome transcriptomic profiling of control embryos and |
|
GPR182 does not trigger downstream signaling in response to CXCL12. |
|
GPR182 modulates CXCR4 signaling through internalizing CXCL12. |
|
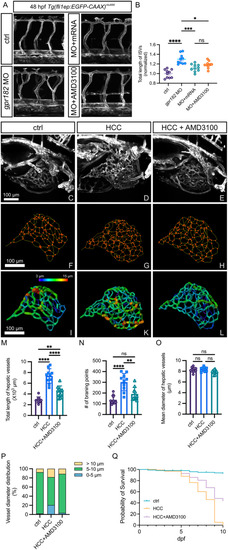
Inhibition of CXCR4 signaling normalizes the vasculature in |
|
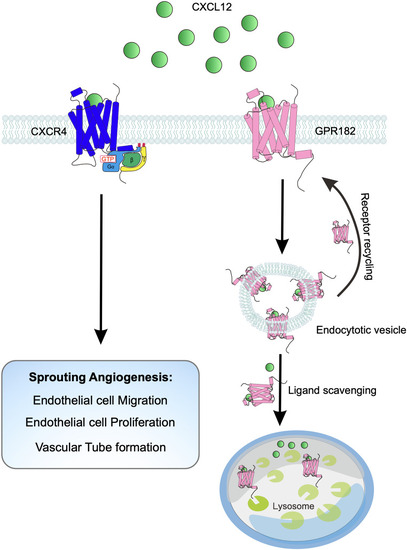
Schematic overview of GPR182’s role in regulating sprouting angiogenesis. This illustration depicts how GPR182 functions as a chemokine scavenger to modulate the CXCL12/CXCR4 signaling axis, thereby controlling the process of sprouting angiogenesis |