- Title
-
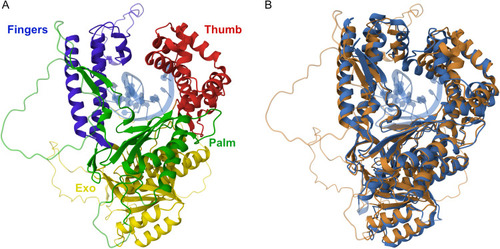
Zebrafish Polymerase Theta and human Polymerase Theta: Orthologues with homologous function
- Authors
- Thomas, C., Green, S., Kimball, L., Schmidtke, I.R., Rothwell, L., Griffin, M., Par, I., Schobel, S., Palacio, Y., Towle-Weicksel, J.B., Weicksel, S.E.
- Source
- Full text @ PLoS One
|
|
|
(A) SDS-PAGE of purified hPol θ PD and zPol θ PD. Expression and purification of zPol θ PD was the same as hPol θ PD as described in the Materials and Methods. For each sample, approximately 56–60 pmol of cleaved, purified protein were loaded on a 10% SDS PAGE and Coomassie stained. Both hPol θ and zPol θ PD fragments migrate to approximately 90 kDa as expected. (B) Circular dichroism spectra of 3 μM hPol θ PD (solid line) and zPol θ PD (dashed) proteins in 10 mM Potassium Phosphate buffer. Samples were scanned from 190 to 280 nm. (C) The same samples were heated from 20–90°C and ellipticity measured at 222 nm. |
|
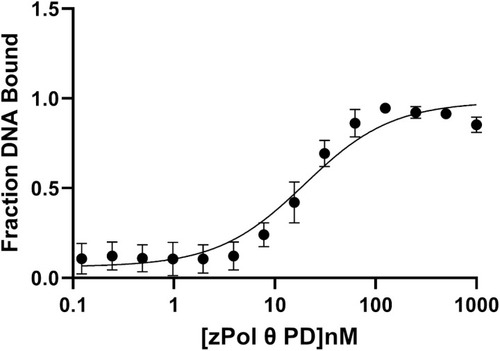
zPol θ PD was titrated from 0–1000 nM against 10 nM 25/40 dsDNA. Bound and unbound products were separated on a 6% non-denaturing gel and quantified using ImageQuant. KD(DNA) was mathematically calculated using |
|
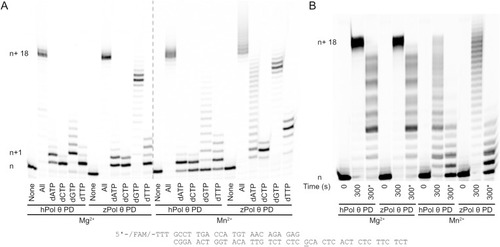
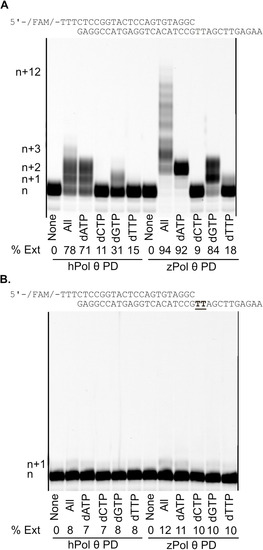
Denaturing gel showing primer extension dsDNA substrate with MgCl2 and MnCl2. (A) Under steady-state conditions 50 nM hPol θ or zPol θ PD proteins were preincubated with 200 nM 25/40 dsDNA and combined with either 10 mM MgCl2 or MnCl2 for 5 minutes at 37°C. (B) The assay was repeated under steady-state conditions, with 10 nM Pol θ and 25 nM 25/40 dsDNA. Reactions were initiated with 100 µM dNTPs, 10 mM MgCl2 or MnCl2, and 75-fold excess of unlabeled 25/40 dsDNA for 5 minutes at 37°C (indicated by a *). DNA extension products were separated on a denaturing gel and visualized on a Typhoon scanner. Each n+1 band represents an extension of one nucleotide following the DNA template as described above. n+1 would represent either correct nucleotide incorporation of dCTP opposite a templating G (underlined) or a misincorporation event of dATP, dGTP, or dTTP opposite templating G. Each subsequent band is another nucleotide extension with a maximum template-dependent extension of n+18. Bands migrating higher than n+18 represent de novo synthesis. |
|
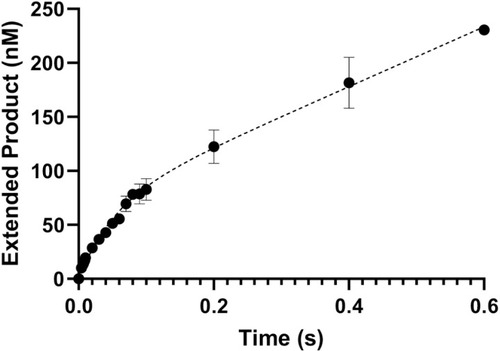
ZPol θ PD (100 nM) was preincubated with 300 nM 25/40 dsDNA. The DNA/Pol θ PD complex was rapidly combined with 100 μM dCTP (correct nucleotide) and 10 mM MgCl2. Reactions were carried out at 37°C and quenched with 0.5 M EDTA. Products were separated on a denaturing gel and quantified with ImageQuant software. Data were fit to a biphasic burst equation to obtain observed |
|
Non-Denaturing gel demonstrating alignment and extension of ssDNA. zPol θ PD (20 nM) was preincubated with 30 nM 5’-FAM 12-mer ssDNA in reaction buffer. Samples either had all nucleotides (+dNTP) or no nucleotides (-dNTP) added and the ternary complex was incubated for 45 minutes at 37°C. Reactions were stopped and products separated on a 12% Native PAGE. The gel was visualized on a Typhoon scanner. MMEJ products are indicated, as well as smaller snap-back products indicated by the bracket. |
|
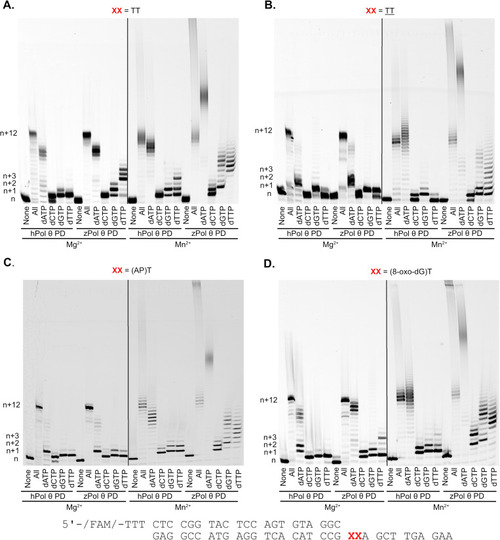
Denaturing gel demonstrating primer extension on damaged dsDNA substrates with MgCl2 and MnCl2.As described in the Materials and Methods, 200 nM hPol θ and zPol θ PDs were preincubated with 50 nM DNA substrate, (A) Undamaged (TT), (B) CPD ( |
|
|