- Title
-
Pancreatic exocrine damage induces beta cell stress in zebrafish larvae
- Authors
- Faraj, N., Hoogaars, W.M.H., Duinkerken, B.H.P., Wolters, A.H.G., Kats, K., Dekkers, M.C., Zaldumbide, A., Giepmans, B.N.G.
- Source
- Full text @ Diabetologia
|
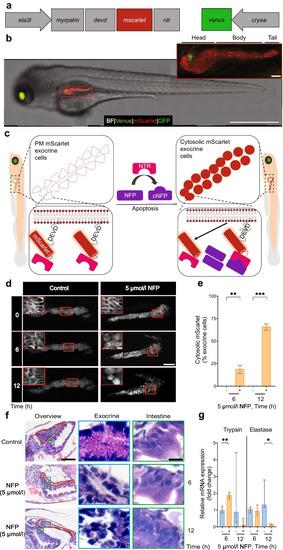
Modulation of exocrine damage in a transgenic zebrafish. ( |
|
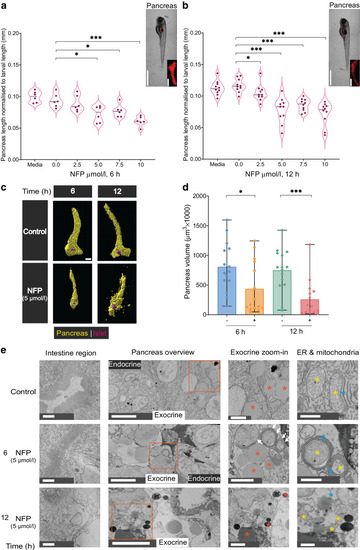
Exocrine damage induces morphological changes and reduces pancreas volume in zebrafish. ( |
|
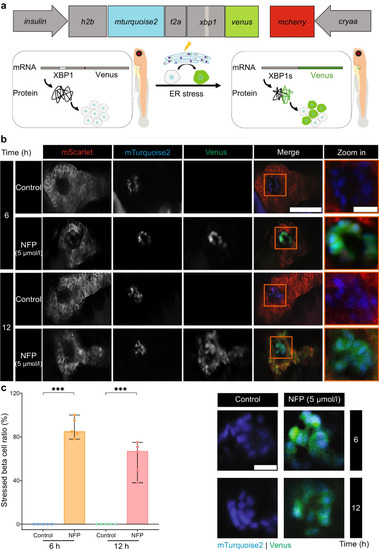
Exocrine damage induces ER stress in beta cells. ( |
|
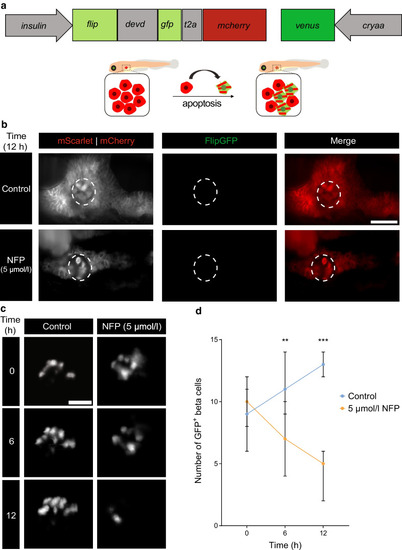
Reduced number of GFP+ beta cells with absence of apoptosis post-exocrine damage. ( |
|
Reduced islet volume and decreased numbers of insulin-producing beta cells following exocrine damage. ( |