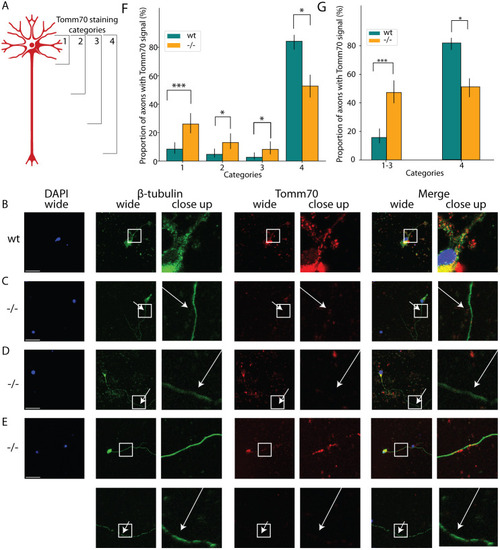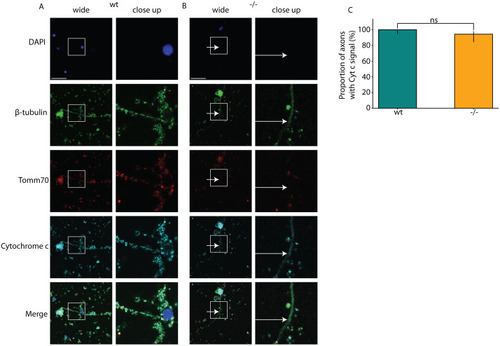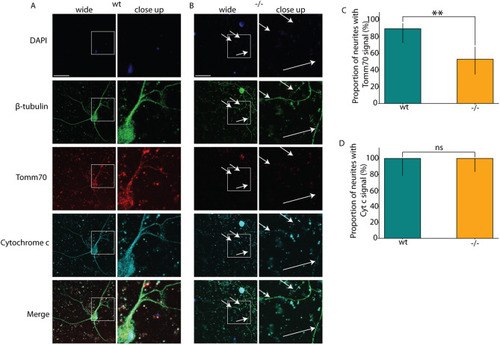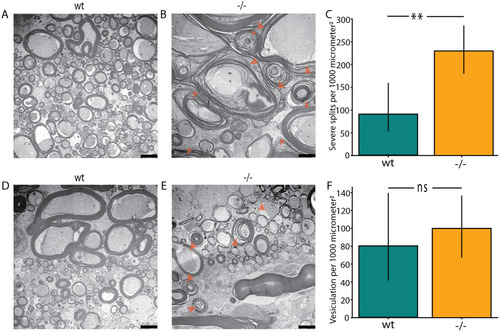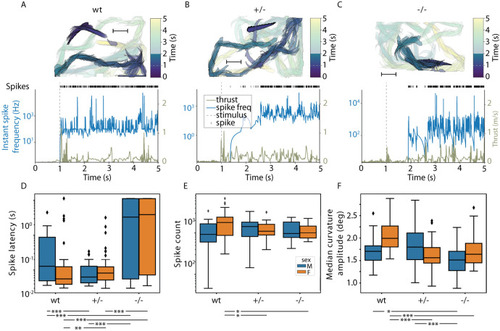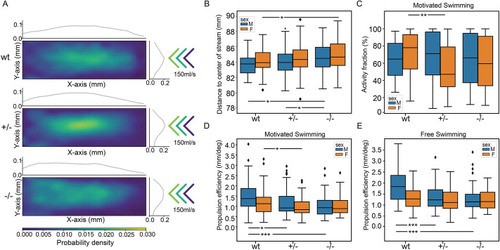
Locomotion defects in tomm70 mutant fish. (A) Two-dimensional heat map with marginal histogram showing all possible locations of female fish in the setup. Blue represents low location probability; yellow represents high location probability. Although wt and heterozygous mutant female fish remain in the centre of the stream, the homozygous female mutants avoid it and reside at a longer distance from the centre of the stream. (B) Box plot of mean distance of fish to the centre of stream, propulsion efficiency and activity fraction. The black line represents the median of all individuals, the box displays the upper and lower quartiles, the whiskers denote 1.5 times the interquartile distance, and the diamonds mark outliers. There is a significant increase in the mean distance of homozygous mutant male and female fish from the centre of stream compared to that for their wt counterparts. (C) Activity is defined as the percentage of video frames in which swimming velocity exceeded 0.025 m/s. Decrease in the activity of heterozygous and homozygous female fish compared to that of female wt fish was observed during motivated swimming trials. There is no change in the activity fraction of male mutants. The activity fraction is normalised to the time period of recording the activity of the fish, which is 30 s for motivated swimming. (D,E) Reduction in the propulsion efficiency of heterozygous and homozygous mutant males and females compared to that of wt males and females in motivated (D) and free-swimming (E) trials. Propulsion efficiency is defined as the number of bends produced by the fish per distance covered in a given time period. N=59 (female wt), 150 (female +/−) and 65 (female −/−), and N=46 (male wt), 91 (male +/−) and 84 (male −/−), in free-swimming trials; N=59 (female wt), 150 (female +/−) and 66 (female −/−), and N=45 (male wt), 96 (male +/−) and 81 (male −/−), in motivated swimming trials; and female N=45 (female wt), 91 (female +/−) and 57 (female −/−), and male N=46 (male wt), 60 (male +/−) and 75 (male −/−), in counter-current trials. Statistical significance was tested using Fisher's permutation test. *P<0.05, **P<0.01, ***P<0.001.
|