- Title
-
Transcriptomic neuron types vary topographically in function and morphology
- Authors
- Shainer, I., Kappel, J.M., Laurell, E., Donovan, J.C., Schneider, M.W., Kuehn, E., Arnold-Ammer, I., Stemmer, M., Larsch, J., Baier, H.
- Source
- Full text @ Nature
|
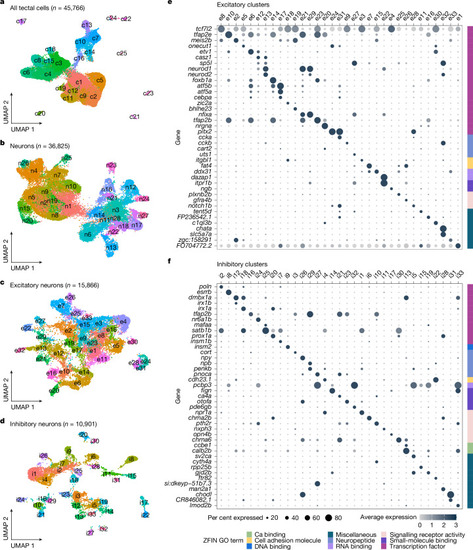
scRNA-seq of the OT reveals a multitude of neuronal types. |
|
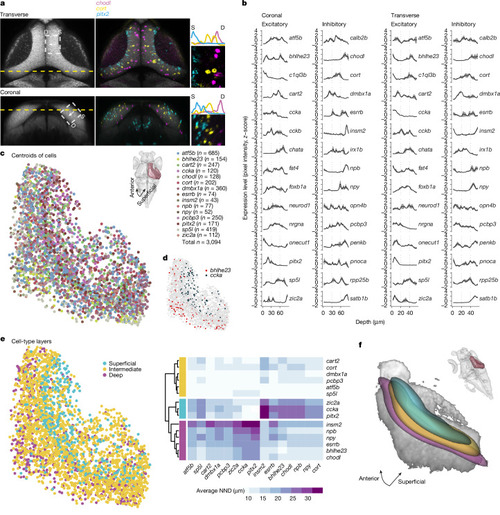
Cell bodies of transcriptomic clusters form anatomical layers in the SPV orthogonal to the retinotopic map. |
|
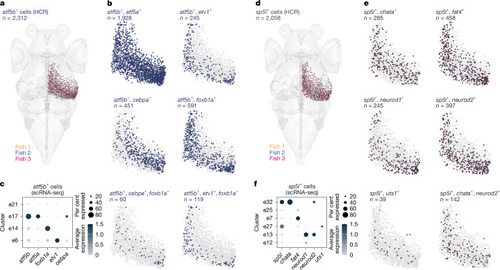
Combinatorial expression of t-type marker genes separates subtypes along the anatomical axes of the OT. |
|
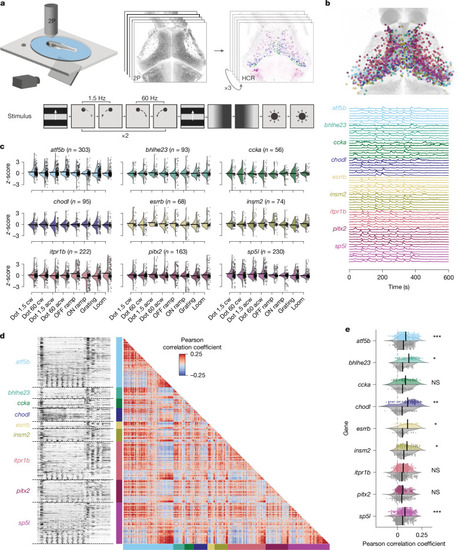
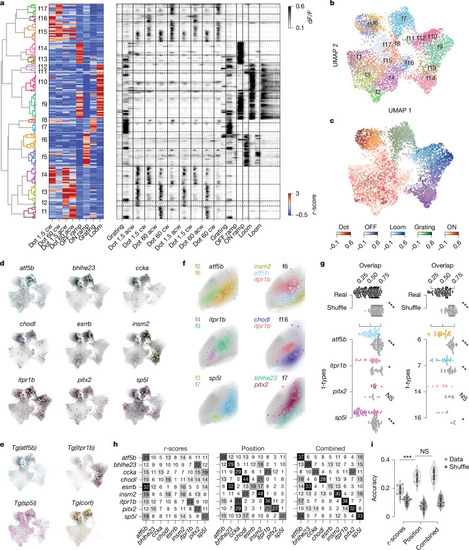
Neurons of a specific t-type show diverse visual responses and form coherent functional subclusters. |
|
Localization in functional and anatomical space varies between t-types. |
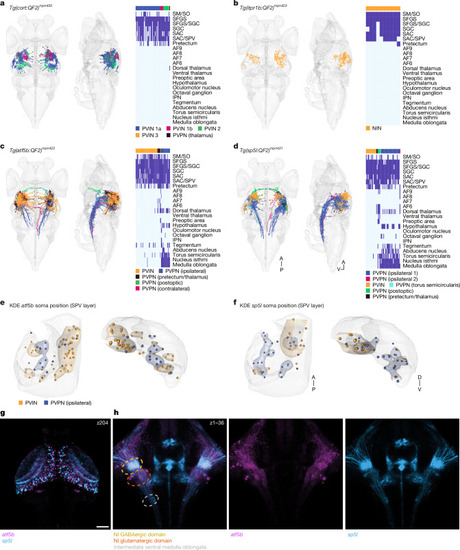
|
Neurons of a specific t-type may assume distinct morphologies and projection patterns. |