- Title
-
The administration of exogenous HSP47 as a collagen specific therapeutic approach
- Authors
- Besio, R., Garibaldi, N., Sala, A., Tonelli, F., Aresi, C., Maffioli, E., Casali, C., Torriani, C., Biggiogera, M., Villani, S., Rossi, A., Tedeschi, G., Forlino, A.
- Source
- Full text @ JCI Insight
|
Recombinant heat shock protein 47 (rHSP47) is taken up by human fibroblasts. ( |
|
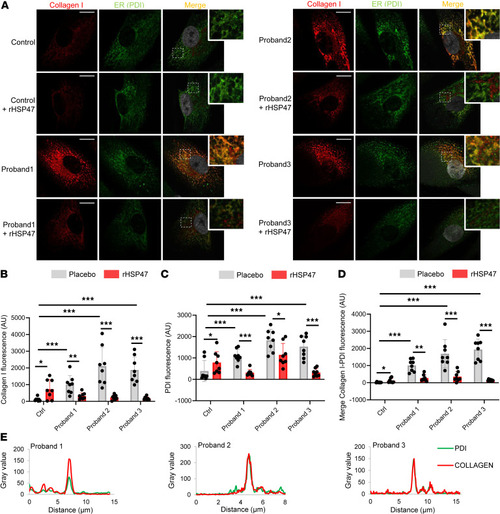
Treatment with rHSP47 reduces intracellular procollagen retention. ( |
|
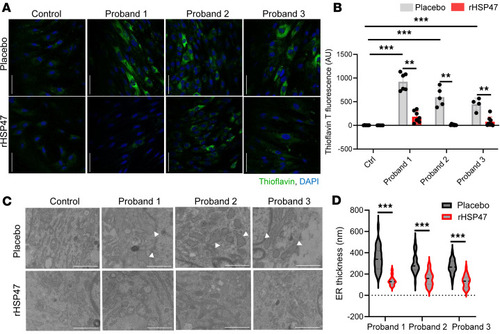
rHSP47 ameliorates cellular homeostasis. ER proteostasis was evaluated by thioflavin T (ThT) labeling of protein aggregates in osteogenesis imperfecta (OI) proband and control fibroblasts treated for 16 hours with 0.5 μM rHSP47 or with placebo. ( |
|
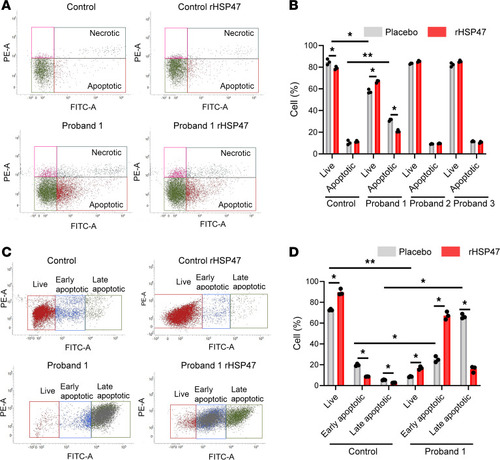
rHSP47 modulates cell apoptosis. ( |
|
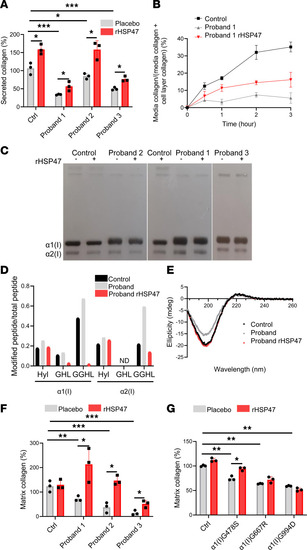
rHSP47 increases collagen secretion, reduces collagen overmodifications, and enhances collagen I deposition in the ECM. ( |
|
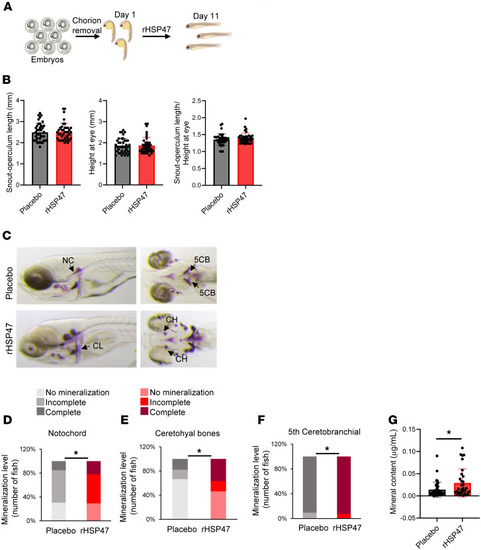
Treatment with rHSP47 ameliorates zebrafish ( |