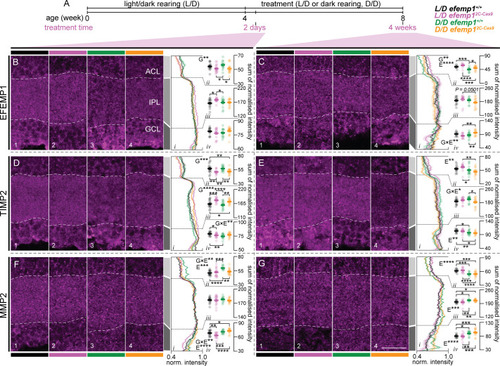
Distribution of EFEMP1, TIMP2 and MMP2 in the inner retina of efemp1+/+ and efemp12C-Cas9 fish after 2 days or 4 weeks of normal L/D cycle or D/D rearing. (A) efemp1+/+ and efemp12C-Cas9 fish were reared under standard L/D condition to 4 wpf and then were under L/D- or D/D-rearing to up to 8 wpf (4 weeks of treatment). Eyes for histological analysis were sampled after 2 days or 4 weeks of treatment. (B,C) Immunostaining for EFEMP1 to analyze its distribution in the inner retina, including amacrine cell layer (ACL), inner plexiform layer (IPL) and ganglion cell layer (GCL), for L/D-reared efemp1+/+, L/D-reared efemp12C-Cas9, D/D-reared efemp1+/+ and D/D-reared efemp12C-Cas9 fish after 2 days (B; n=16, 16, 14 and 14 retinae, respectively) and 4 weeks (C; n=12, 12, 15 and 14 retinae, respectively) of treatment. White dashed lines in micrographs highlight the borders of the IPL. Average normalized expression across the inner retina is shown in Bi and Ci and summed normalized expression (normalized intensity) for (Bii and Cii) ACL, (Biii and Ciii) IPL and (Biv and Civ) GCL was quantified for each eye. (D,E) Immunostaining for TIMP2 in the inner retina for L/D-reared efemp1+/+, L/D-reared efemp12C-Cas9, D/D-reared efemp1+/+ and D/D-reared efemp12C-Cas9 fish after 2 days (D; n=16, 16, 16 and 15 retinae, respectively) and 4 weeks (E; n=13, 14, 15 and 14 retinae, respectively) of treatment. Average normalized expression is shown in Di and Ei and summed normalized expression for (Dii and Eii) ACL, (Diii and Eiii) IPL and (Div and Eiv) GCL. (F,G) Immunostaining for MMP2 for L/D-reared efemp1+/+, L/D-reared efemp12C-Cas9, D/D-reared efemp1+/+ and D/D-reared efemp12C-Cas9 fish after 2 days (F; n=16, 16, 12 and 15 retinae, respectively) and 4 weeks (G; n=13, 13, 12 and 15 retinae, respectively) of treatment. Average normalized expression is shown in Fi and Gi and summed normalized expression for (Fii and Gii) ACL, (Fiii and Giii) IPL and (Fiv and Giv) GCL. Scale bar in G represents 20 μm and all images share the same scale. Group data are shown as mean±s.e.m. Two-way ANOVA and Fisher's LSD post-hoc tests were performed. Significant main effects of genotypes (G), rearing conditions (E) and their interactions (G×E) are indicated in the graphs. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
|